Abstract
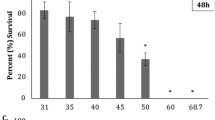
Temperature and salinity are important environmental factors affecting the normal functioning of marine animals, particularly animals such as sea urchins living in shallow waters and tide pools. Here, we evaluated the effect of different combinations of temperature and salinity on early embryos of the endemic New Zealand sea urchin Evechinus chloroticus. Animals were collected at Matheson’s Bay (36º18′17′′S; 174º47′51′′E) in north-eastern New Zealand in February 2013. Embryos were exposed to five salinities (29, 31, 34, 35 and 37 ppt) and two temperatures (18 and 21 °C) during the first 24 h of development. Low salinity (29 ppt) affected all parameters (fertilization, development rate, gastrulation and normal development), with ca. 50 % of embryos surviving at 29 ppt, whereas seawater temperature only affected development rate and gastrulation. An increase in temperature from 18 to 21 °C minimized the negative effect of low salinity (≤31 ppt) on development rate and gastrulation of E. chloroticus. Overall, the results of this study suggest that early embryos of E. chloroticus have developmental plasticity to withstand reductions in salinity up to 29 ppt; however, it is still unknown whether the surviving embryos will be able to complete larval development at low salinities, particularly whether the embryos and larvae are carried into extreme environments such as estuaries where salinity is even lower. Multistressor studies are very important for climate change research as multiple environmental factors will act together in the wild, having major consequences for development and recruitment of marine invertebrates.






Similar content being viewed by others
References
Allen JD, Pechenik JA (2010) Understanding the effects of low salinity on fertilization success and early development in the sand dollar Echinarachnius parma. Biol Bull 218:189–199
Antonie CR (2003) Effects of low salinity on Evechinus Chloroticus Valenciennes. MSc Thesis, University of Otago, Dunedin
Armstrong AF, Blackburn HN, Allen JD (2013) A novel report of hatching plasticity in the phylum Echinodermata. Am Nat 181:264–272. doi:10.1086/668829
Azad KA, McKinley S, Pearce CM (2010) Factors influencing the growth and survival of larval and juvenile echinoids. Rev Aquac 2:121–137. doi:10.1111/j.1753-5131.2010.01030.x
Barker M (2007) Ecology of Evechinus chloroticus. In: John ML (ed) Developments in aquaculture and fisheries science. Elsevier, Amsterdam, pp 319–338
Bressan M, Marin M, Brunetti R (1995) Influence of temperature and salinity on embryonic development of Paracentrotus lividus (Lmk, 1816). Hydrobiologia 304:175–184. doi:10.1007/bf02329312
Byrne M, Przeslawski R (2013) Multistressor impacts of warming and acidification of the ocean on marine invertebrates’ life histories. Integr Comp Biol 53:582–596. doi:10.1093/icb/ict049
Byrne M, Ho M, Selvakumaraswamy P, Nguyen HD, Symon AD, Davis AR (2009) Temperature, but not pH, compromises sea urchin fertilization and early development under near-future climate change scenarios. Philos Trans R Soc B Biol Sci 276:1883–1888. doi:10.2307/30244023
Byrne M, Soars N, Selvakumaraswamy P, Dworjanyn SA, Davis AR (2010) Sea urchin fertilization in a warm, acidified and high pCO2 ocean across a range of sperm densities. Mar Environ Res 69:234–239. doi:10.1016/j.marenvres.2009.10.014
Byrne M, Selvakumaraswamy P, Ho MA, Woolsey E, Nguyen HD (2011) Sea urchin development in a global change hotspot, potential for southerly migration of thermotolerant propagules. Deep Sea Res Part II 58:712–719. doi:10.1016/j.dsr2.2010.06.010
Byrne M, Foo S, Soars NA, Wolfe KDL, Nguyen HD, Hardy N, Dworjanyn SA (2013) Ocean warming will mitigate the effects of acidification on calcifying sea urchin larvae (Heliocidaris tuberculata) from the Australian global warming hot spot. J Exp Mar Biol Ecol 448:250–257. doi:10.1016/j.jembe.2013.07.016
Cameron R, Boidron-Metairon I, Monterrosa O (1985) Does the embryonic response to temperature and salinity by four species of Caribbean sea urchins parallel the reproductive synchrony? In: Proc 5th Int Coral Reef Congr 5: pp 273–278
Carballeira C, Martín-Díaz L, DelValls TA (2011) Influence of salinity on fertilization and larval development toxicity tests with two species of sea urchin. Mar Environ Res 72:196–203. doi:10.1016/j.marenvres.2011.08.008
Castro P, Huber ME (2005) Marine biology. McGraw-Hill, Boston
Ciapa B, Philippe L (2013) Intracellular and extracellular pH and Ca are bound to control mitosis in the early sea urchin embryo via ERK and MPF activities. PLoS One 8:e66113. doi:10.1371/journal.pone.0066113
Clark D, Lamare M, Barker M (2009) Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: a comparison among a tropical, temperate, and a polar species. Mar Biol 156:1125–1137. doi:10.1007/s00227-009-1155-8
Cowart D, Ulrich P, Miller D, Marsh A (2009) Salinity sensitivity of early embryos of the Antarctic sea urchin, Sterechinus neumayeri. Polar Biol 32:435–441. doi:10.1007/s00300-008-0536-7
Delorme NJ, Sewell MA (2013) Temperature limits to early development of the New Zealand sea urchin Evechinus chloroticus (Valenciennes, 1846). J Therm Biol 38:218–224. doi:10.1016/j.jtherbio.2013.02.007
Diaz-Perez L, Carpizo-Ituarte E (2011) Effect of thermal stress on survival and delay of metamorphosis in larvae of the purple sea urchin Strongylocentrotus purpuratus. Cienc Mar 37:403–414
Dix T (1969) Larval life span of the echinoid Evechinus chloroticus (val.). New Zeal J Mar Fresh 3:13–16. doi:10.1080/00288330.1969.9515273
Dix T (1970) Biology of Evechinus chloroticus (Echinoidea: Echinometridae) from different localities: 1. General. New Zeal J Mar Fresh 4:91–116. doi:10.1080/00288330.1970.9515331
Fenwick G, Horning D (1980) Echinodermata of the Snares Islands, southern New Zealand. New Zeal J Mar Fresh 14:437–445. doi:10.1080/00288330.1980.9515888
Folt CL, Chen CY, Moore MV, Burnaford J (1999) Synergism and antagonism among multiple stressors. Limnol Oceanogr 44(3):864–877
Franke ES (2005) Aspects of fertilization ecology in Evechinus chloroticus and Coscinasterias muricata. PhD thesis, University of Auckland, Auckland
Fujisawa H (1995) Variation in embryonic temperature sensitivity among groups of the sea urchin, Hemicentrotus pulcherrimus, which differ in their habitats. Zool Sci 12:583–589. doi:10.2108/zsj.12.583
Garner DM (1969) The seasonal range of sea temperature on the New Zealand shelf. New Zeal J Mar Fresh 3:201–208. doi:10.1080/00288330.1969.9515289
George SB, Walker D (2007) Short-term fluctuation in salinity promotes rapid larval development and metamorphosis in Dendraster excentricus. J Exp Mar Biol Ecol 349:113–130. doi:10.1016/j.jembe.2007.05.010
Greenwood PJ, Bennett T (1981) Some effects of temperature-salinity combinations on the early development of the sea urchin Parechinus angulosus (Leske). Fertilization. J Exp Mar Biol Ecol 51:119–131. doi:10.1016/0022-0981(81)90124-6
Hardy NA, Lamare M, Uthicke S, Wolfe K, Doo S, Dworjanyn S, Byrne M (2014) Thermal tolerance of early development in tropical and temperate sea urchins: inferences for the tropicalization of eastern Australia. Mar Biol 161:395–409. doi:10.1007/s00227-013-2344-z
IPCC (2014) Climate change 2014: Impact, adaptation and vulnerability. Working Group II contribution to the IPCC 5th Assessment Report
Kashenko S (2006) The combined effect of temperature and salinity on development of the sea star. Russ J Mar Biol 32:37–44. doi:10.1134/s1063074006010056
Kashenko S (2007) Adaptive responses of embryos and larvae of the heart-shaped sea urchin Echinocardium cordatum to temperature and salinity changes. Russ J Mar Biol 33:381–390. doi:10.1134/S1063074007060041
Kashenko S (2009) Effects of extreme changes of sea water temperature and salinity on the development of the sand dollar Scaphechinus mirabilis. Russ J Mar Biol 35:422–430. doi:10.1134/S1063074009050083
Kinne O (1964) The effects of temperature and salinity on marine and brackish water animals. II. Salinity and temperature-salinity combinations. Oceanogr Mar Biol Annu Rev 2:281–339
Lalli CM, Parsons TR (1997) Biological oceanography: an introduction. Butterworth Heinemann, Oxford
Lawrence JM (1975) The effect of temperature-salinity combinations on the functional well- being of adult Lytechinus variegatus (Lamarck) (Echinodermata, Echinoidea). J Exp Mar Biol Ecol 18:271–275. doi:10.1016/0022-0981(75)90111-2
Li L, Li Q, Sun X, Kong L (2011) Effects of temperature and salinity on larval growth, survival, and development of the sea cucumber Apostichopus japonicus. N Am J Aquacult 73:296–303. doi:10.1080/15222055.2011.598373
Metaxas A (1998) The effect of salinity on larval survival and development in the sea urchin Echinometra lucunter. Invertebr Reprod Dev 34:323–330. doi:10.1080/07924259.1998.9652667
Metaxas A, Young CM (1998) Behaviour of echinoid larvae around sharp haloclines: effects of the salinity gradient and dietary conditioning. Mar Biol 131:443–459. doi:10.1007/s002270050337
Moore HB (1966) General biology of echinoderms. In: Boolootian RA (ed) Physiology of Echinodermata. Interscience Publishers, New York, pp 1–48
Morgan SG (1995) Life and death in the plankton: larval mortality and adaptation. In: McEdward LR (ed) Ecology of marine invertebrate larvae. CRC Press, New York
Nakagawa S, Cuthill IC (2007) Effect size, confidence interval and statistical significance: a practical guide for biologists. Biol Rev 82:591–605. doi:10.1111/j.1469-185x.2007.00027.x
Nottage RAC, Wratt DS, Bornman JF, Jones K (2010) Climate change adaptation in New Zealand: future scenarios and some sectoral perspectives. New Zealand Climate Change Centre, Wellington
O’ Connor MI, Bruno JF, Gaines SD, Halpern BS, Lester SE, Kinlan BP, Weiss JM (2007) Temperature control of larval dispersal and the implications for marine ecology, evolution, and conservation. Proc Natl Acad Sci USA 104:1266–1271. doi:10.1073/pnas.0603422104
Peake B, Walls D, Gibbs M (2001) Spatial variations in the levels of nutrients, chlorophyll a, and dissolved oxygen in summer and winter in Doubtful Sound, New Zealand. New Zeal J Mar Fresh 35:681–694. doi:10.1080/00288330.2001.9517035
Pia TS, Johnson T, George SB (2012) Salinity-induced morphological changes in Pisaster ochraceus (Echinodermata: Asteroidea) larvae. J Plankton Res 34:590–601. doi:10.1093/plankt/fbs032
Renwick J, Mullan B, Wilcocks L, Zammit C, Sturman J, Baisden T, Keller L, Kirschbaum M, Meason D, Harrison D, Verkerk G, Cooke A, Marshall P, Clark A (2013) Four degrees of global warming: effects on the New Zealand primary sector. Ministry of Primary Industries, Wellington
Roller RA, Stickle WB (1985) Effects of salinity on larval tolerance and early developmental rates of four species of echinoderms. Can J Zool 63:1531–1538. doi:10.1139/z85-227
Roller RA, Stickle WB (1993) Effects of temperature and salinity acclimation of adults on larval survival, physiology, and early development of Lytechinus variegatus (Echinodermata: Echinoidea). Mar Biol 116:583–591. doi:10.1007/BF00355477
Roller RA, Stickle WB (1994) Effects of adult salinity acclimation on larval survival and early development of Strongylocentrotus droebachiensis and Strongylocentrotus pallidus (Echinodermata: Echinoidea). Can J Zool 72:1931–1939. doi:10.1139/z94-262
Russell MP (2013) Echinoderm responses to variation in salinity. Adv Mar Biol 66:171–212. doi:10.1016/B978-0-12-408096-6.00003-1
Sameoto JA, Metaxas A (2008) Can salinity-induced mortality explain larval vertical distribution with respect to a halocline? Biol Bull 214(3):329–338
Schiel D, Kingsford MJ, Choat JH (1986) Depth distribution and abundance of benthic organisms and fishes at the subtropical Kermadec Islands. New Zeal J Mar Fresh 20:521–535. doi:10.1080/00288330.1986.9516173
Sewell MA, Young CM (1999) Temperature limits to fertilization and early development in the tropical sea urchin Echinometra lucunter. J Exp Mar Biol Ecol 236:291–305. doi:10.1016/S0022-0981(98)00210-X
Shanks AL (1995) Mechanisms of cross-shelf dispersal of larval invertebrates and fish. In: McEdward LR (ed) Ecology of marine invertebrate larvae. CRC Press, New York
Sharp DJ (2002) Cell division: MAST Sails through mitosis. Curr Biol 12:R585–R587. doi:10.1016/S0960-9822(02)01098-9
Sharp DJ, Rogers GC, Scholey JM (2000) Microtubule motors in mitosis. Nature 407:41–47. doi:10.1038/35024000
Thorson G (1950) Reproductive and larval ecology of marine bottom invertebrates. Biol Rev 25:1–45. doi:10.1111/j.1469-185X.1950.tb00585.x
Trowbridge CD (1994) Life at the edge: population dynamics and salinity tolerance of a high intertidal, pool- dwelling ascoglossan opisthobranch on New Zealand rocky shores. J Exp Mar Biol Ecol 182:65–84. doi:10.1016/0022-0981(94)90211-9
Walker MM (1984) Larval life span, larval settlement, and early growth of Evechinus chloroticus (Valenciennes). New Zeal J Mar Fresh 18:393–397. doi:10.1080/00288330.1984.9516060
Walker J, Vaughan M (2013) Marine water quality annual report: 2011. Auckland Council technical report, TR2013/031. Auckland Council, Auckland
Willmer P (1999) Environmental physiology of animals. Blackwell, Massachusetts
Zeldis JR (2004) New and remineralised nutrient supply and ecosystem metabolism on the northeastern New Zealand continental shelf. Cont Shelf Res 24:563–581. doi:10.1016/j.csr.2003.11.008
Zeldis JR, Smith SV (1999) Water, salt and nutrient budgets for Hauraki Gulf New Zealand. In: Smith SV, Crossland CJ (eds) Australasian Estuarine Systems: carbon, nitrogen and phosphorus fluxes, LOICZ Reports and Studies No. 12, 182 pp, LOICZ IPO, Texel, The Netherlands
Zeldis JR, Walters RA, Greig MJN, Image K (2004) Circulation over the northeastern New Zealand continental slope, shelf and adjacent Hauraki Gulf, during spring and summer. Cont Shelf Res 24:543–561. doi:10.1016/j.csr.2003.11.007
Acknowledgments
Thanks to Errol Murray, Peter Browne, for helping with the experimental setup, and to Brady Doak and Richard Taylor for providing diving equipment. Thanks to Leonardo Zamora for helping with animal collection, spawning and sampling. NJD was financially supported by a Chilean Government scholarship (Becas-Chile, National Commission for Scientific and Technological Research, CONICYT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Byrne.
Rights and permissions
About this article
Cite this article
Delorme, N.J., Sewell, M.A. Temperature and salinity: two climate change stressors affecting early development of the New Zealand sea urchin Evechinus chloroticus . Mar Biol 161, 1999–2009 (2014). https://doi.org/10.1007/s00227-014-2480-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2480-0