Abstract
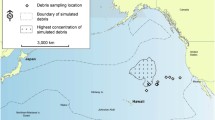
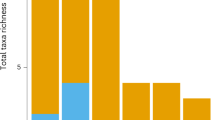
Plastic and other anthropogenic debris (e.g., rubber, tar) augment natural floating substrates (e.g., algal rafts, pumice) in the open ocean, allowing “islands” of substrate-associated organisms to persist in an otherwise unsuitable habitat. We examined a total of 242 debris objects collected in the eastern Pacific in 2009 and 2011 (32–39°N, 130–142°W) and the western Pacific in 2012 (19–41°N, 143–156°E). Here, we ask: (a) What taxa are associated with plastic rafts in the North Pacific? and (b) Does the number of taxa associated with plastic debris vary with the size of the debris “island?” We documented 95 rafting taxa from 11 phyla. We identified several potentially invasive plastic-associated rafting taxa, including the coral pathogen Halofolliculina spp. In concordance with classic species–area curves, the number of rafting taxa was positively correlated with the size of the raft. Our findings suggest that diversity patterns on plastic debris are compatible with the concept of island biogeography.




Similar content being viewed by others
References
Aliani S, Molcard A (2003) Hitch-hiking on floating marine debris: macrobenthic species in the Western Mediterranean Sea. Hydrobiologia 503:59–67. doi:10.1023/B:HYDR.0000008480.95045.26
Andrady AL (2011) Microplastics in the marine environment. Mar Pollut Bull 62:1596–1605. doi:10.1016/j.marpolbul.2011.05.030
Astudillo JC, Bravo M, Dumont CP, Thiel M (2009) Detached aquaculture buoys in the SE Pacific: potential dispersal vehicles for associated organisms. Aquat Biol 5:219–231. doi:10.3354/ab00151
Barnes DKA (2002) Invasions by marine life on plastic debris. Nature 416:808–809. doi:10.1038/416808a
Barnes DKA, Fraser KPP (2003) Rafting by five phyla on man-made flotsam in the Southern Ocean. Mar Ecol Prog Ser 262:289–291. doi:10.3354/meps262289
Barnes DKA, Milner P (2005) Drifting plastic and its consequences for sessile organism dispersal in the Atlantic Ocean. Mar Biol 146:815–825. doi:10.1007/s00227-004-1474-8
Barnes DKA, Galgani F, Thompson RC, Barlaz M (2009) Accumulation and fragmentation of plastic debris in global environments. Philos T Roy Soc B 364:1985–1998. doi:10.1098/rstb.2008.0205
Boero F, Bucci C, Colucci AMR et al (2007) Obelia (Cnidaria, Hydrozoa, Campanulariidae): a microphagous, filter-feeding medusa. Mar Ecol 28:178–183. doi:10.1111/j.1439-0485.2007.00164.x
Bravo M, Astudillo JC, Lancellotti D et al (2011) Rafting on abiotic substrata: properties of floating items and their influence on community succession. Mar Ecol Prog Ser 439:1–17. doi:10.3354/meps09344
Brown DM, Cheng L (1981) New net for sampling the ocean surface. Mar Ecol Prog Ser 5:225–227. doi:10.3354/meps005225
Bryan SE, Cook AG, Evans JP et al (2012) Rapid, long-distance dispersal by pumice rafting. PLoS ONE 7:e40583. doi:10.1371/journal.pone.0040583
Caine EA (1977) Feeding mechanisms and possible resource partitioning of the Caprellidae (Crustacea: Amphipoda) from Puget Sound, USA. Mar Biol 42:331–336. doi:10.1007/BF00402195
Carpenter EJ, Smith KL (1972) Plastics on the Sargasso Sea surface. Science 175:1240. doi:10.1126/science.175.4027.1240
Carson HS, Nerheim MS, Carroll KA, Eriksen M (2013) The plastic-associated microorganisms of the North Pacific Gyre. Mar Pollut Bull 75:126–132. doi:10.1016/j.marpolbul.2013.07.054
Carter MC, Bishop JDD, Evans NJ, Wood CA (2010) Environmental influences on the formation and germination of hibernacula in the brackish-water bryozoan Victorella pavida Saville Kent, 1870 (Ctenostomata: Victorellidae). J Exp Mar Biol Ecol 383:89–95. doi:10.1016/j.jembe.2009.11.012
Choong HHC, Calder DR (2013) Sertularella mutsuensis Stechow, 1931 (Cnidaria: Hydrozoa: Sertulariidae) from Japanese tsunami debris: systematics and evidence for transoceanic dispersal. BioInvasions Rec 2:33–38
Clarkin E, Maggs CA, Allcock AL, Johnson MP (2012) Environment, not characteristics of individual algal rafts, affects composition of rafting invertebrate assemblages in Irish coastal waters. Mar Ecol Prog Ser 470:31–40
Croquer A, Bastidas Carolina, Lipscomb D (2006) Folliculinid ciliates: a new threat to Caribbean corals? Dis Aquat Org 69:75–78. doi:10.3354/dao069075
Dameron OJ, Parke M, Albins MA, Brainard R (2007) Marine debris accumulation in the Northwestern Hawaiian Islands: an examination of rates and processes. Mar Pollut Bull 54:423–433. doi:10.1016/j.marpolbul.2006.11.019
Davidson TM (2012) Boring crustaceans damage polystyrene floats under docks polluting marine waters with microplastic. Mar Pollut Bull 64:1821–1828. doi:10.1016/j.marpolbul.2012.06.005
Dempster T, Kingsford M (2003) Homing of pelagic fish to fish aggregation devices (FADs): the role of sensory cues. Mar Ecol Prog Ser 258:213–222. doi:10.3354/meps258213
Derraik JGB (2002) The pollution of the marine environment by plastic debris: a review. Mar Pollut Bull 44:842–852. doi:10.1016/S0025-326X(02)00220-5
Development Core Team R (2012) R: a language and environment for statistical computing. Austria, Vienna
Donlan CJ, Nelson PA (2003) Observations of invertebrate colonized flotsam in the eastern tropical Pacific, with a discussion of rafting. B Mar Sci 72:231–240
Emerson W, Chaney H (1995) A zoogeographic review of the Cypraeidae (Mollusca, Gastropoda) occurring in the eastern Pacific Ocean. Veliger 38:8–21
Evans F (1958) Growth and maturity of the barnacles Lepas hillii and Lepas anatifera. Nature 182:1245–1246. doi:10.1038/1821245b0
Farrapeira CMR (2011) Invertebrados macrobentônicos detectados na costa brasileira transportados por resíduos flutuantes sólidos abiogênicos. Revista da Gestão Costeira Integrada 11:85–96
Fofonoff P, Ruiz G, Steves B, Carlton J (2012) National Exotic Marine and Estuarine Species Information System. In: Smithsonian Environmental Research Center. http://invasions.si.edu/nemesis/. Accessed 26 Oct 2012
Gewin V (2013) Tsunami triggers invasion concerns. Nature 495:13–14. doi:10.1038/495013a
Godwin LS, Harris L, Charette A, Moffitt R (2008) The marine invertebrate species associated with the biofouling of derelict fishing gear in the Pāpahanaumokuākea–Marine National Monument: a focus on marine non-native species transport. p 26
Goldstein MC, Goodwin DS (2013) Gooseneck barnacles (Lepas spp.) ingest microplastic debris in the North Pacific Subtropical Gyre. PeerJ 1:184. doi:10.7717/peerj.184
Goldstein MC, Rosenberg M, Cheng L (2012) Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol Lett 8:817–820. doi:10.1098/rsbl.2012.0298
Goldstein MC, Titmus AJ, Ford M (2013) Scales of spatial heterogeneity of plastic marine debris in the northeast Pacific ocean. PLoS ONE 8:e80020. doi:10.1371/journal.pone.0080020
Hammer J, Kraak HS Michiel, Parsons John R (2012) Plastics in the marine environment: the dark side of a modern gift. Rev Environ Contam T 220:1–44. doi:10.1007/978-1-4614-3414-6_1
He F, Legendre P (1996) On species-area relations. Am Nat 148:719–737
Hidalgo-Ruz V, Gutow L, Thompson RC, Thiel M (2012) Microplastics in the marine environment: a review of the methods used for identification and quantification. Environ Sci Technol 46:3060–3075. doi:10.1021/es2031505
Hirosaki Y (1960) Observations and experiments on the behavior of fishes toward floating objects in aquarium. J Fac Sci Hokkaido Univ 14:320–326
Hobday AJ (2000) Persistence and transport of fauna on drifting kelp (Macrocystis pyrifera (L.) C. Agardh) rafts in the Southern California Bight. J Exp Mar Biol Ecol 253:75–96. doi:10.1016/S0022-0981(00)00250-1
Hoeksema BW, Roos PJ, Cadee GC (2012) Trans-Atlantic rafting by the brooding reef coral Favia fragum on man-made flotsam. Mar Ecol Prog Ser 445:209–218. doi:10.3354/meps09460
Ibrahim S, Ambak MA, Shamsudin L, Samsudin MZ (1996) Importance of fish aggregating devices (FADs) as substrates for food organisms of fish. Fish Res 27:265–273. doi:10.1016/0165-7836(96)00473-0
Ingólfsson A (1995) Floating clumps of seaweed around Iceland: natural microcosms and a means of dispersal for shore fauna. Mar Biol 122:13–21. doi:10.1007/BF00349273
Jacobsen JK, Massey L, Gulland F (2010) Fatal ingestion of floating net debris by two sperm whales (Physeter macrocephalus). Mar Pollut Bull 60:765–767. doi:10.1016/j.marpolbul.2010.03.008
Karl DM, Bidigare RR, Letelier RM (2001) Long-term changes in plankton community structure and productivity in the North Pacific Subtropical Gyre: the domain shift hypothesis. Deep Sea Res Pt II 48:1449–1470. doi:10.1016/S0967-0645(00)00149-1
Law KL, Moret-Ferguson S, Maximenko NA et al (2010) Plastic accumulation in the North Atlantic Subtropical Gyre. Science 329:1185–1188. doi:10.1126/science.1192321
Lebreton LC-M, Borrero JC (2013) Modeling the transport and accumulation floating debris generated by the 11 March 2011 Tohoku tsunami. Mar Pollut Bull 66:53–58. doi:10.1016/j.marpolbul.2012.11.013
Lesser MP, Shumway SE, Cucci T, Smith J (1992) Impact of fouling organisms on mussel rope culture: interspecific competition for food among suspension-feeding invertebrates. J Exp Mar Biol Ecol 165:91–102. doi:10.1016/0022-0981(92)90291-H
Lomolino MV (2000) Ecology’s most general, yet protean pattern: the species-area relationship. J Biogeogr 27:17–26
Lovely EC (2005) The life history of Phoxichilidium tubulariae (Pycnogonida: Phoxichilidiidae). Northeastern Nat 12:77–92. doi:10.1656/1092-6194(2005)012[0077:TLHOPT]2.0.CO;2
MacArthur RH, Wilson EO (1963) An equilibrium theory of insular zoogeography. Evolution 17:373–387
Majer AP, Vedolin MC, Turra A (2012) Plastic pellets as oviposition site and means of dispersal for the ocean-skater insect Halobates. Mar Pollut Bull 64:1143–1147. doi:10.1016/j.marpolbul.2012.03.029
Maso M, Garces E, Pages F, Camp J (2003) Drifting plastic debris as a potential vector for dispersing harmful algal bloom (HAB) species. Sci Mar 67:107–111
Matassa CM, Trussell GC (2011) Landscape of fear influences the relative importance of consumptive and nonconsumptive predator effects. Ecology 92:2258–2266. doi:10.1890/11-0424.1
Matthews DC (1963) Hawaiian records of folliculinids (Protozoa) from submerged wood. Pac Sci 17:438–443
McDermid KJ, McMullen TL (2004) Quantitative analysis of small-plastic debris on beaches in the Hawaiian archipelago. Mar Pollut Bull 48:790–794
Mook DH (1981) Removal of suspended particles by fouling communities. Mar Ecol Prog Ser 5:279–281
Moret-Ferguson S, Law KL, Proskurowski G et al (2010) The size, mass, and composition of plastic debris in the western North Atlantic Ocean. Mar Pollut Bull 60:1873–1878. doi:10.1016/j.marpolbul.2010.07.020
Nelson PA (2003) Marine fish assemblages associated with fish aggregating devices (FADs): effects of fish removal, FAD size, fouling communities, and prior recruits. Fish B-NOAA 101:835–850
Palmer C, Gates R (2010) Skeletal eroding band in Hawaiian corals. Coral Reefs 29:469. doi:10.1007/s00338-010-0597-2
Pham PH, Jung J, Lumsden JS et al (2012) The potential of waste items in aquatic environments to act as fomites for viral haemorrhagic septicaemia virus. J Fish Dis 35:73–77. doi:10.1111/j.1365-2761.2011.01323.x
Pister B (2009) Urban marine ecology in southern California: the ability of riprap structures to serve as rocky intertidal habitat. Mar Biol 156:861–873. doi:10.1007/s00227-009-1130-4
Pratt MC (2008) Living where the flow is right: how flow affects feeding in bryozoans. Integr Comp Biol 48:808–822. doi:10.1093/icb/icn052
Rasband WS (2012) ImageJ. U. S. National Institutes of Health, Bethesda MD. http://imagej.nih.gov/ij/
Riemann-Zürneck K (1998) How sessile are sea anemones? A review of free-living forms in the Actiniaria (Cnidaria: Anthozoa). Mar Ecol 19:247–261. doi:10.1111/j.1439-0485.1998.tb00466.x
Rodriguez SR, Ojeda FP, Inestrosa NC (1993) Settlement of benthic marine invertebrates. Mar Ecol Prog Ser 97:193–207
Rodriguez S, Cróquer A, Guzmán H, Bastidas C (2009) A mechanism of transmission and factors affecting coral susceptibility to Halofolliculina sp. infection. Coral Reefs 28:67–77. doi:10.1007/s00338-008-0419-y
Ryan PG (2013) A simple technique for counting marine debris at sea reveals steep litter gradients between the Straits of Malacca and the Bay of Bengal. Mar Pollut Bull 69:128–136. doi:10.1016/j.marpolbul.2013.01.016
Sheldon RW, Prakash A, Sutcliffe WH (1972) The size distribution of particles in the ocean. Limnol Oceangr 17:327–340
Simberloff D (1976) Experimental zoogeography of islands: effects of island size. Ecology 57:629–648
Thiel M, Gutow L (2005a) The ecology of rafting in the marine environment I: the floating substrata. Oceanogr Mar Biol 42:181–263
Thiel M, Gutow L (2005b) The ecology of rafting in the marine environment II: the rafting organisms and community. Oceanogr Mar Biol 43:279–418
Thiel M, Haye PA (2006) The ecology of rafting in the marine environment III: biogeographical and evolutionary consequences. Oceanogr Mar Biol 44:323–429
Titmus AJ, Hyrenbach KD (2011) Habitat associations of floating debris and marine birds in the North East Pacific Ocean at coarse and meso spatial scales. Mar Pollut Bull 62:2496–2506. doi:10.1016/j.marpolbul.2011.08.007
Tsikhon-Lukanina EA, Reznichenko OG, Nikolaeva GG (2001) Ecology of invertebrates on the oceanic floating substrata in the northwest Pacific ocean. Oceanology 41:525–530
Tyrrell MC, Byers JE (2007) Do artificial substrates favor nonindigenous fouling species over native species? J Exp Mar Biol Ecol 342:54–60
U.S. Environmental Protection Agency (2011) Marine Debris in the North Pacific: A summary of existing information and identification of data gaps. p 23
Vassilopoulou V, Siapatis A, Christides G, Bekas P (2004) The biology and ecology of juvenile pilotfish (Naucrates ductor) associated with fish aggregating devices (FADs) in eastern Mediterranean waters. Mediterr Mar Sci 5:61–70. doi:10.12681/mms.211
Venrick EL, Backman TW, Bartram WC et al (1973) Man-made objects on the surface of the central North Pacific Ocean. Nature 241:271. doi:10.1038/241271a0
Whitehead TO, Biccard A, Griffiths CL (2011) South African pelagic goose barnacles (Cirripedia, Thoracica): substratum preferences and influence of plastic debris on abundance and distribution. Crustaceana 84:635–649. doi:10.1163/001121611X574290
Winston JE, Gregory MR, Stevens L (1997) Encrusters, epibionts, and other biota associated with pelagic plastics: a review of biological, environmental, and conservation issues. In: Coe JM, Rogers DB (eds) Marine debris: sources, impact and solutions. Springer, New York, pp 81–98
Wong CS, Green DR, Cretney WJ (1974) Quantitative tar and plastic waste distributions in Pacific Ocean. Nature 247:30–32. doi:10.1038/247030a0
Ye S, Andrady AL (1991) Fouling of floating plastic debris under Biscayne Bay exposure conditions. Mar Pollut Bull 22:608–613. doi:10.1016/0025-326X(91)90249-R
Zettler ER, Mincer TJ, Amaral-Zettler LA (2013) Life in the “Plastisphere”: microbial communities on plastic marine debris. Environ Sci Technol 47:7137–7146. doi:10.1021/es401288x
Acknowledgments
Funding for the 2009 SEAPLEX cruise was provided by University of California Ship Funds, Project Kaisei/Ocean Voyages Institute, AWIS-San Diego, and NSF IGERT Grant No. 0333444. The 2011 and 2012 expeditions were made possible through collaboration between the 5 Gyres Institute, Algalita Marine Research Institute, and the University of Hawaii, Hilo. The Sea Dragon was made available by Pangaea Explorations. M.C.G. was supported by NSF GK-12 Grant No. 0841407 and donations from Jim and Kris McMillan, Jeffrey and Marcy Krinsk, Lyn and Norman Lear, Ellis Wyer, and the Petersen Charitable Foundation. Funding for H.S.C. was provided by the Will J. Reid Foundation through a grant to K. McDermid. Laboratory supplies, support, and some analytical equipment for the 2009 samples were supplied by the SIO Pelagic Invertebrate Collection and the California Current Ecosystem LTER site supported by NSF. Many thanks to the captain and crew of the R/V New Horizon and Sea Dragon for their assistance in debris collection and many other logistics. We are grateful to H. Cha, M. DeMaintenon, M. Forrest, E. Moore, L. Sala, A. Townsend, and J. Winston for their assistance with taxonomy and to S. Strutt for her work in the laboratory. Comments from L. Gutow, M.R. Landry, M.D. Ohman, M. Thiel, and an anonymous reviewer significantly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goldstein, M.C., Carson, H.S. & Eriksen, M. Relationship of diversity and habitat area in North Pacific plastic-associated rafting communities. Mar Biol 161, 1441–1453 (2014). https://doi.org/10.1007/s00227-014-2432-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2432-8