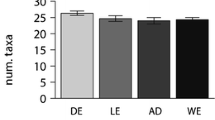
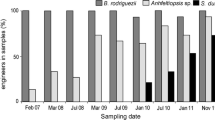
Abstract
This study examined the effects of a guild of micrograzing harpacticoid copepods (dominated by two species of Paradactylopodia sp. nov. and one species of Scutellidium sp. nov.) and a mesograzing periwinkle, Afrolittorina praetermissa, on the early recruitment of intertidal macroalgae on a wave-exposed, rocky shore. This is the first study, to our knowledge, to examine the effects of micrograzers (<500 μm) on intertidal macroalgal recruitment. Data showed that microscopic harpacticoid copepods altered the assemblages and reduced the densities of several macroalgal taxa, while A. praetermissa changed the assemblages and reduced both the density and number of macroalgal taxa. Recruitment of encrusting coralline algae was actually higher in copepod inclusions than exclusions, suggesting that copepods may be beneficial to the recruitment of this algal group. These results contribute to the understanding of grazing as a factor causing high mortality of algal recruits, but also highlight the need for more studies that examine the effects of micro- and mesograzers on the distribution and abundance of macroalgae.





Similar content being viewed by others
References
Anderson MJ, Gorley RN, Clarke KR (2008) PERMANOVA+ for PRIMER: guide to software and statistical methods. PRIMER-E, Plymouth, p 214
Andrew NL, Underwood AJ (1993) Density-dependent foraging in the sea urchin Centrostephanus rodgersii on shallow subtidal reefs in New South Wales, Australia. Mar Ecol Prog Ser 99:89–98
Ang POJ, De Wreede RE (1992) Density-dependence in a population of Fucus distichus. Mar Ecol Prog Ser 90:169–181
Bellgrove A (1998) Recruitment of intertidal macroalgae on a wave-exposed rocky coast. Monash University, Clayton
Bellgrove A, Clayton MN, Quinn GP (2004) An integrated study of the temporal and spatial variation in the supply of propagules, recruitment and assemblages of intertidal macroalgae on a wave-exposed rocky coast, Victoria, Australia. J Exp Mar Biol Ecol 310:207–225
Bennett S, Bellwood DR (2011) Latitudinal variation in macroalgal consumption by fishes on the Great Barrier Reef. Mar Ecol Prog Ser 426:241–252
Brawley SH (1992) Mesoherbivores. In: John DM, Hawkins SJ, Price JH (eds) Plant-animal interactions in the marine benthos. Clarendon Press, Oxford, pp 235–263
Brawley SH, Adey WH (1981a) The effect of micrograzers on algal community structure in a coral reef microcosm. Mar Biol 61:167–177
Brawley SH, Adey WH (1981b) Micrograzers may affect macroalgal density. Nature 292:177
Brawley SH, Fei XG (1987) Studies of mesoherbivory in aquaria and an unbarricaded mariculture farm on the Chinese coast. J Phycol 23:614–623
Burkepile DE, Hay ME (2010) Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. PLoS One 5:e8963
Buschmann AH, Bravo A (1990) Intertidal amphipods as potential dispersal agents of carpospores of Irideae laminarioides (Gigartinales, Rhodophyta). J Phycol 26:417–420
Cheal A, Emslie M, Miller I, Sweatman H (2012) The distribution of herbivorous fishes on the Great Barrier Reef. Mar Biol 159:1143–1154
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Aust J Ecol 18:117–143
Connell SD, Russell BD, Turner DJ, Shepherd SA, Kildea T, Miller D, Airoldi L, Cheshire A (2008) Recovering a lost baseline: missing kelp forests from a metropolitan coast. Mar Ecol Prog Ser 360:63–72
Duffy JE, Hay ME (1994) Herbivore resistance to seaweed chemical defense: the roles of mobility and predation risk. Ecology 75:1304–1319
Duffy JE, Hay ME (2000) Strong impacts of grazing amphipods on the organization of a benthic community. Ecol Monogr 70:237–263
Dunmore RA, Schiel DR (2003) Demography, competitive interactions and grazing effects of intertidal limpets in southern New Zealand. J Exp Mar Biol Ecol 288:17–38
Fletcher WJ (1987) Interactions among subtidal Australian sea urchins, gastropods, and algae: effects of experimental removals. Ecol Monogr 57:89–109
Giakoumi S, Cebrian E, Kokkoris GD, Ballesteros E, Sala E (2012) Relationships between fish, sea urchins and macroalgae: the structure of shallow rocky sublittoral communities in the Cyclades, Eastern Mediterranean. Estuar Coast Shelf Sci 109:1–10
Harley CDG, Anderson KM, Demes KW, Jorve JP, Kordas RL, Coyle TA, Graham MH (2012) Effects pf climate change on global seaweed communities. J Phycol 48:1064–1078
Hawkins SJ, Hartnoll RG (1983) Grazing of intertidal algae by marine invertebrates. Oceanogr Mar Biol Annu Rev 21:195–282
Hixon MA, Brostoff WN (1996) Succession and herbivory—effects of differential fish grazing on Hawaiian coral-reef algae. Ecol Monogr 66:67–90
Jacobucci GB, Tanaka MO, Leite FPP (2009) Temporal variation of amphipod assemblages associated with Sargassum filipendula (Phaeophyta) and its epiphytes in a subtropical shore. Aquat Ecol 43:1031–1040
Jernakoff P (1983) Factors affecting the recruitment of algae in a midshore region dominated by barnacles. J Exp Mar Biol Ecol 67:17–31
Kendrick GA (1994) Effects of propagule settlement density and adult canopy on survival of recruits of Sargassum spp. (Sargassaceae: Phaeophyta). Mar Ecol Prog Ser 103:129–140
Keough MJ, Downes BJ (1982) Recruitment of marine invertebrates: the role of active larval choices and early mortality. Oecologia 54:348–352
Kitting CL (1989) Algal recruitment among dense invertebrate herbivores; do intertidal molluscan “grazers” enhance their algal foods. Bull Mar Sci 45:550
Kruskal JB (1964) Non-parametric multidimensional scaling: a numerical method. Psychometrika 29:115–129
Lazo L, Markham JH, Chapman ARO (1994) Herbivory and harvesting: effects on sexual recruitment and vegetative modules of Ascophyllum nodosum. Ophelia 40:95–113
Livore JP, Connell SD (2012) Fine-scale effects of sedentary urchins on canopy and understory algae. J Exp Mar Biol Ecol 411:66–69
Lubchenco J (1978) Plant species diversity in a marine intertidal community: importance of herbivore food preference and algal competitive abilities. Am Nat 112:23–39
Lubchenco J (1982) Effects of grazers and algal competitors on fucoid colonization in tide pools. J Phycol 18:544–550
Mangialajo L, Chiantore M, Cattaneo-Vietti R (2008) Loss of fucoid algae along a gradient of urbanisation, and structure of benthic assemblages. Mar Ecol Prog Ser 358:63–74
McClanahan TR, Kurtis JD (1991) Population regulation of the rock-boring sea urchin Echinometra mathaei (de Blainville). J Exp Mar Biol Ecol 147:121–146
McClanahan TR, Sala E (1997) A Mediterranean rocky-bottom ecosystem fisheries model. Ecol Model 104:145–164
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Box S, Stoffle RW, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101
O’Leary JK, McClanahan TR (2010) Trophic cascades result in large-scale coralline algae loss through differential grazer effects. Ecology 91:3584–3597
Paine RT, Vadas RL (1969) The effects of grazing by sea urchins, Strongylocentrotus spp. on benthic algal populations. Limnol Oceanogr 14:710–719
Parker T, Johnson C, Chapman ARO (1993) Gammarid amphipods and littorinid snails have significant but different effects on algal succession in littoral fringe tidepools. Ophelia 38:69–88
Petraitis PS (1992) Effects of body size and water temperature on grazing rates of 4 intertidal gastropods. Aust J Ecol 17:409–414
Poore AGB (1994) Selective herbivory by amphipods inhabiting the brown alga Zonaria angustata. Mar Ecol Prog Ser 107:113–123
Poore AGB, Campbell AH, Steinberg PD (2009) Natural densities of mesograzers fail to limit growth of macroalgae or their epiphytes in a temperate algal bed. J Ecol 97:164–175
Povey A, Keough MJ (1991) Effects of trampling on plant and animal populations on rocky shores. Oikos 61:355–368
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Reed DC (1990) The effects of variable settlement and early competition on patterns of kelp recruitment. Ecology 71:776–787
Santelices B, Paya I (1989) Digestion survival of algae: some ecological comparisons between free spores and propagules in fecal pellets. J Phycol 25:693–699
Santelices B, Correa J, Avila M (1983) Benthic algal spores surviving digestion by sea urchins. J Exp Mar Biol Ecol 70:263–269
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Underwood AJ, Kennelly SJ (1990) Ecology of marine algae on rocky shores and subtidal reefs in temperate Australia. Hydrobiologia 192:3–20
Vermeij MJA, van der Heijden RA, Olthuis JG, Marhaver KL, Smith JE, Visser PM (2013) Survival and dispersal of turf algae and macroalgae consumed by herbivorous coral reef fishes. Oecologia 171:417–425
Acknowledgments
Access to study sites within the Mornington Peninsula National Park (now part of the Port Phillip Heads Marine National Park) was gratefully appreciated. Dr. B. Burton, Dr. M. Wheatley, Dr. M. Burd and P. Druce are much appreciated for their assistance with fieldwork. K. Miles, R. Watson and P. Domelow provided technical assistance and advice throughout. Comments from Drs. M. Wheatley, M.N. Aoki and A.R.O. Chapman improved the manuscript. This research was supported by an Australian Postgraduate Award. Prof M.N. Clayton is thanked and acknowledged for her supervision throughout this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Bellgrove, A., Monk, J. & Quinn, G.P. Effects of micro- and mesograzers on intertidal macroalgal recruitment. Mar Biol 161, 1207–1216 (2014). https://doi.org/10.1007/s00227-014-2411-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-014-2411-0