Abstract
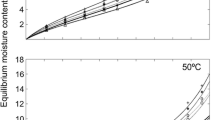
For industrial processes, it is important to study the hygroscopicity and thermodynamic properties of juvenile and mature wood. Samples of Abies pinsapo Boiss. collected in the natural areas of the species in Spain were used to study these properties in both types of wood. The equilibrium moisture contents were obtained, and the 15, 35 and 50 °C isotherms were plotted following the Guggenheim–Anderson–Boer–Dent model. The thermodynamic parameters were calculated using the integration method of the Clausius–Clapeyron equation. Chemical analyses, infrared spectra and X-ray diffractograms were applied to assess chemical modifications and possible changes in the cell wall structure. The chemical composition of the mature wood shows a decrease in the lignin and hemicelluloses content and an increase in the extracts and α-cellulose. The sorption isotherms for the three temperatures studied are higher in the mature wood than in the juvenile wood. Causes of this include the higher content of α-cellulose, the higher crystallinity index and the shorter crystallite length in the mature wood. No difference was found between the juvenile and mature wood in relation to the point of inflexion where the multilayer starts to predominate over the monolayer (approximately 30 %). In terms of the thermodynamic properties, the heat involved is greater in desorption than in adsorption, and more heat is involved in the mature wood than in the juvenile wood.



Similar content being viewed by others
References
AENOR (1982) Determinación de los extractos solubles en agua. Standard UNE 57013. Asociación Española de Normalización, Madrid
AENOR (1986) Determinación del contenido de lignina insoluble en ácido. Standard UNE 57100. Asociación Española de Normalización, Madrid
AENOR (1999) Determinación de materias soluble en acetona. Standard UNE-EN ISO 14453. Asociación Española de Normalización, Madrid
Andersson S, Wikberg H, Pesonen E, Maunu SL, Serimaa R (2004) Studies of crystallinity of Scots pine and Norway spruce cellulose. Trees Struct Funct 18:346–353
Avramidis S (1997) The basics of sorption, international conference on wood–water relations, Copenhagen, Denmark, 16–17 June, pp 1–16
Bertaud F, Holmbom B (2004) Chemical composition of earlywood and latewood in Norway spruce heartwood, sapwood and transition zone wood. Wood Sci Technol 38:245–256
Bonarski J, Olek W (2011) Application of the crystalline volume fraction for characterizing the ultrastructural organization of wood. Cellulose 18:223–235
Browning BL (1963) The chemistry of wood. Interscience, New York
Chirife J, Timmermann EO, Iglesias HA, Boquet R (1992) Some features of the parameter-K of the Gab equation as applied to sorption isotherms of selected food materials. J Food Eng 15:75–82
Christensen GN, Kelsey KE (1959) The rate of sorption of water vapor by wood. Holz Roh Werkst 17:178–188
Engelund ET, Thygesen LG, Svensson S, Hill CAS (2013) A critical discussion of the physics of wood–water interactions. Wood Sci Technol 47:141–161
Esteban LG, Fernández FG, Casasus AG, de Palacios PD, Gril J (2006) Comparison of the hygroscopic behaviour of 205-year-old and recently cut juvenile wood from Pinus sylvestris L. Ann For Sci 63:309–317
Esteban LG, de Palacios P, Fernández FG, Guindeo A, Cano NN (2008a) Sorption and thermodynamic properties of old and new Pinus sylvestris wood. Wood Fiber Sci 40:111–121
Esteban LG, de Palacios P, Fernández FG, Guindeo A, Conde M, Baonza V (2008b) Sorption and thermodynamic properties of juvenile Pinus sylvestris L. wood after 103 years of submersion. Holzforschung 62:745–751
Esteban LG, de Palacios P, Fernández FG, Martín JA, Génova M, Fernández-Golfín JI (2009) Sorption and thermodynamic properties of buried juvenile Pinus sylvestris L. wood aged 1170 ± 40 BP. Wood Sci Technol 43:679–690
Esteban LG, de Palacios P, Fernández FG, García-Amorena I (2010) Effects of burial of Quercus spp. wood aged 5910 ± 250 BP on sorption and thermodynamic properties. Int Biodeterior Biodegrad 64:371–377
Fernandez FG, Esteban LG, de Palacios P, Simon C, Garcia-Iruela A, de la Fuente J (2014) Sorption and thermodynamic properties of Terminalia superba Engl. & Diels. and Triplochiton scleroxylon K. Schum. through the 15, 35 and 50 °C sorption isotherms. Eur J Wood Prod 72:99–106
Hill CAS, Norton AJ, Newman G (2010) The water vapour sorption properties of Sitka spruce determined using a dynamic vapour sorption apparatus. Wood Sci Technol 44:497–514
Hill C, Moore J, Jalaludin Z, Leveneu M, Mahrdt E (2011) Influence of earlywood/latewood and ring position upon water vapour sorption properties of Sitka spruce. Int Wood Prod J 2:1–19
Jahan MS, Mun SP (2005) Effect of tree age on the cellulose structure of Nalita wood (Trema orientalis). Wood Sci Technol 39:367–373
Jalaludin Z, Hill CAS, Samsi HW, Husain H, Xie YJ (2010) Analysis of water vapour sorption of oleo-thermal modified wood of Acacia mangium and Endospermum malaccense by a parallel exponential kinetics model and according to the Hailwood-Horrobin model. Holzforschung 64:763–770
Jowitt R, Wagstaffe PJ (1989) The certification of water content of microcrystalline cellulose (MCC) at 10 water activities. Commission of the European Communities. Community Bureau of Reference, EUR 12429, EN, Brussels, Belgium
Kadita S, Yamada T, Suzuki M (1961) Studies on rheological properties of wood I. Effect of moisture content on the dynamic Young’s modulus of wood. Mokuzai Gakkaishi 7:29–33
Kollmann F (1951) Technologie des Holzes und der Holzwerkstoffe, (Technology of wood and wood based products), vol I. Springer, Berlin
Kouhila M, Kechaou N, Otmani M, Fliyou M, Lahsasni S (2002) Experimental study of sorption isotherms and drying kinetics of Moroccan Eucalyptus globulus. Dry Technol 20:2027–2039
Lenth CA, Kamke FA (2001) Equilibrium moisture content of wood in high-temperature pressurized environments. Wood Fiber Sci 33:104–118
Lequin S, Chassagne D, Karbowiak T, Gougeon R, Brachais L, Bellat JP (2010) Adsorption equilibria of water vapor on cork. J Agric Food Chem 58:3438–3445
Lionetto F, Del Sole R, Cannoletta D, Vasapollo G, Maffezzoli A (2012) Monitoring wood degradation during weathering by cellulose crystallinity. Materials 5:1910–1922
Maheswari CU, Reddy KO, Muzenda E, Guduri BR, Rajulu AV (2012) Extraction and characterization of cellulose microfibrils from agricultural residue—Cocos nucifera L. Biomass Bioenerg 46:555–563
Maskan M, Gogus F (1997) The fitting of various models to water sorption isotherms of pistachio nut paste. J Food Eng 33:227–237
McMillin CW (1968) Chemical composition of Loblolly pine wood as related to specific gravity, growth rate, and distance from pith. Wood Sci Technol 2:233–240
McMinn WAM, Magee TRA (2003) Thermodynamic properties of moisture sorption of potato. J Food Eng 60:157–165
Militz H, Busetto D, Hapla F (2003) Investigation on natural durability and sorption properties of Italian Chestnut (Castanea sativa Mill.) from coppice stands. Holz Roh Werkst 61:133–141
Moreira R, Chenlo F, Vazquez MJ, Camean P (2005) Sorption isotherms of turnip top leaves and stems in the temperature range from 298 to 328 K. J Food Eng 71:193–199
Neimsuwan T, Wang S, Taylor AM, Rials TG (2008) Statics and kinetics of water vapor sorption of small loblolly pine samples. Wood Sci Technol 42:493–506
Papadopoulos AN, Hill CAS (2003) The sorption of water vapour by anhydride modified softwood. Wood Sci Technol 37:221–231
Passialis CN (1997) Physico-chemical characteristics of waterlogged archaeological wood. Holzforschung 51:111–113
Peralta PN, Bangi AP, Lee AWC (1997) Thermodynamics of moisture sorption by the giant-timber bamboo. Holzforschung 51:177–182
Phuong LX, Takayama M, Shida S, Matsumoto Y, Aoyagi T (2007) Determination of the accessible hydroxyl groups in heat-treated Styrax tonkinensis (Pierre) Craib ex Hartwich wood by hydrogen-deuterium exchange and H-2 NMR spectroscopy. Holzforschung 61:488–491
Popescu CM, Hill CAS, Curling S, Ormondroyd G, Xie YJ (2014) The water vapour sorption behaviour of acetylated birch wood: how acetylation affects the sorption isotherm and accessible hydroxyl content. J Mater Sci 49:2362–2371
Quirijns EJ, van Boxtel AJB, van Loon WKP, van Straten G (2005a) An improved experimental and regression methodology for sorption isotherms. J Sci Food Agric 85:175–185
Quirijns EJ, van Boxtel AJB, van Loon WKP, van Straten G (2005b) Sorption isotherms, GAB parameters and isosteric heat of sorption. J Sci Food Agric 85:1805–1814
Rowell RM (2012) Handbook of wood chemistry and wood composites, 2nd edn. CRC Press, Boca Raton
Shupe TF, Hse CY, Choong ET, Groom LH (1997) Differences in some chemical properties of innerwood and outerwood from five silviculturally different loblolly pine stands. Wood Fiber Sci 29:91–97
Siau JF (1995) Wood: Influence of moisture on physical properties. Department of Wood Science and Forest Products, Virginia Polytechnic Institute and State University, Blackburg
Singh PC, Singh RK (1996) Application of GAB model for water sorption isotherms of food products. J Food Process Preserv 20:203–220
Skaar C (1972) Water in wood. Syracuse University Press, Syracuse, NY, 13210, USA
Sumi Y, Hale RD, Meyer JA, Leopold B, Ranby BG (1964) Accessibility of wood and wood carbohydrates measured with tritiated water. Tappi 47:621–624
Tappi standard (2009) Alpha- beta- and gamma-cellulose in pulp, Test Method T203 cm-09
Themelin A, Rebollo J, Thibaut A (1997) Method for defining the behaviour of lignocellulosic produces at sorption: application to tropical wood species, International conference on wood–water relations, Copenhagen, Denmark, 16–17 June, pp 17–32
Thygesen LG, Engelund ET, Hoffmeyer P (2010) Water sorption in wood and modified wood at high values of relative humidity. Part I: results for untreated, acetylated, and furfurylated Norway spruce. Holzforschung 64:315–323
Tsoumis GT (1991) Science and technology of wood. Van Nostrand Reinhold, New York
Viollaz PE, Rovedo CO (1999) Equilibrium sorption isotherms and thermodynamic properties of starch and gluten. J Food Eng 40:287–292
Willems W (2014) The water vapor sorption mechanism and its hysteresis in wood: the water/void mixture postulate. Wood Sci Technol 48:499–518
Wise LE, Murphy M, Daddieco AA (1946) Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Tech Assoc Pap 29:210–218
Yasuda R, Minato K, Norimoto M (1995) Moisture adsorption thermodynamics of chemically modified wood. Holzforschung 49:548–554
Yeh TF, Braun JL, Goldfarb B, Chang HM, Kadla JF (2006) Morphological and chemical variations between juvenile wood, mature wood, and compression wood of loblolly pine (Pinus taeda L.). Holzforschung 60:1–8
Zaihan J, Hill CAS, Curling S, Hashim WS, Hamdan H (2009) Moisture adsorption isotherms of Acacia mangium and Endospermum malaccense using dynamic vapour sorption. J Trop For Sci 21:277–285
Zobel B, Matthias M, Roberds JH, Kellison RC (1968) Moisture content of southern pine trees. Technical report 37 School For Res NC State University Raleigh, NC
Acknowledgments
This study is part of the AGL2009-12801 project of the 2008–2011 Spanish National Plan for Scientific Research, Development and Technological Innovation, funded by the Spanish Ministry of Science and Innovation. The authors are grateful to Forestry Engineers Miguel A. Martín Casillas and José López Quintanilla for assistance in collecting the samples of A. pinsapo.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Esteban, L.G., Simón, C., Fernández, F.G. et al. Juvenile and mature wood of Abies pinsapo Boissier: sorption and thermodynamic properties. Wood Sci Technol 49, 725–738 (2015). https://doi.org/10.1007/s00226-015-0730-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-015-0730-z