Abstract
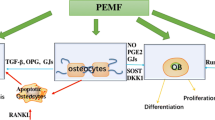
We proposed a three-step strategy to obtain the optimal therapeutic parameters, which is composed of large-scale screening at cellular level, verification in animal experiments, and confirmation by a clinical trial. The objective of the current study was to test the feasibility of our strategy. Newborn rat calvarial osteoblasts were treated by 50 Hz 1.8 mT sinusoidal electromagnetic fields (SEMFs) with 0.5, 1.0, 1.5, 2.0, 2.5, and 3.0 h/days, respectively. The osteogenic differentiation and maturation of the osteoblast were assayed and compared to obtain the optimal duration. One-month-old growing rats were then treated by the same SEMFs with 0.5, 1.5, and 2.5 h/days, respectively, and the peak bone mass was analyzed after 2 months. It was found that the optimal exposure duration to promote the osteogenic differentiation and maturation of osteoblasts was 1.5 h/days, judging by the increasing degrees of ALP activity, calcified nodules formed, the gene and protein expression levels of Runx-2, BMP-2, and Col-I, as well as the expression levels of signaling proteins of the BMP-2/Smad1/5/8 pathway. The highest increase of peak bone mass after 2 months was also obtained by 1.5 h/days, judging by the results of X-ray dual-energy absorptiometry, mechanical property analysis, micro-CT scanning, and serum bone turnover marker examinations. The above results indicated that exposure duration is a determinant for the therapeutic effect of EMFs, and the optimal therapeutic effects only can be obtained by the optimal exposure duration.






Similar content being viewed by others
References
Griffin XL, Warner F, Costa M (2008) The role of electromagnetic stimulation in the management of established non-union of long bone fractures: what is the evidence? Injury 39:419–429
Martinez-Rondanelli A, Martinez JP, Moncada ME, Manzi E, Pinedo CR, Cadavid H (2014) Electromagnetic stimulation as coadjuvant in the healing of diaphyseal femoral fractures: a randomized controlled trial. Colomb Med 45:67–71
Osti L, Del Buono A, Maffulli N (2015) Application of pulsed electromagnetic fields after microfractures to the knee: a mid-term study. Int Orthop 39:1289–1294
Thomas AW, Graham K, Prato FS, McKay J, Forster PM, Moulin D, Chari S (2007) A randomized, double-blind, placebo-controlled clinical trial using a low-frequency magnetic field in the treatment of musculoskeletal chronic pain. Pain Res Manag 12:249–258
Wuschech H, von Hehn U, Mikus E, Funk RH (2015) Effects of PEMF on patients with osteoarthritis: results of a prospective, placebo-controlled, double-blind study. Bioelectromagnetics 36:576–585
Wang R, Wu H, Yang Y, Song M (2016) Effects of electromagnetic fields on osteoporosis: a systematic literature review. Electromagn Biol Med 35:384–390
Saliev T, Mustapova Z, Kulsharova G, Bulanin D, Mikhalovsky S (2014) Therapeutic potential of electromagnetic fields for tissue engineering and wound healing. Cell Prolif 47:485–493
Viganò M, Sansone V, d’Agostino MC, Romeo P, Perucca Orfei C, de Girolamo L (2016) Mesenchymal stem cells as therapeutic target of biophysical stimulation for the treatment of musculoskeletal disorders. J Orthop Surg Res 11:163
Arjmand M, Ardeshirylajimi A, Maghsoudi H, Azadian E (2017) Osteogenic differentiation potential of mesenchymal stem cells cultured on nanofibrous scaffold improved in the presence of pulsed electromagnetic field. J Cell Physiol. https://doi.org/10.1002/jcp.25962
Gupta A, Taly AB, Srivastava A, Kumar S, Thyloth M (2009) Efficacy of pulsed electromagnetic field therapy in healing of pressureulcers: a randomized control trial. Neurol India 57:622–626
Ryang We S, Koog YH, Jeong KI, Wi H (2013) Effects of pulsed electromagnetic fields on knee osteoarthritis: a systematic review. Rheumatology 52:815–824
Hannemann PF, van Wezenbeek MR, Kolkman KA, Twiss EL, Berghmans CH, Dirven PA et al (2014) CT scan-evaluated outcome of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a randomised, multicentre, double-blind, placebo-controlled trial. Bone Joint J 96-B:1070–1076
Maziarz A, Kocan B, Bester M, Budzik S, Cholewa M, Ochiya T et al (2016) How electromagnetic fields can influence adult stem cells:positive and negative impacts. Stem Cell Res Ther 7:54
Zhou J, Ming LG, Ge BF, Wang JQ, Zhu RQ, Wei Z et al (2011) Effects of 50 Hz sinusoidal electromagnetic fields of different intensities on proliferation, differentiation and mineralization potentials of rat osteoblasts. Bone 49:753–761
Luo F, Hou T, Zhang Z, Xie Z, Wu X, Xu J (2012) Effects of pulsed electromagnetic field frequencies on the osteogenic differentiation of human mesenchymal stem cells. Orthopedics 35:e526–e531
Hong JM, Kang KS, Yi HG, Kim SY, Cho DW (2014) Electromagnetically controllable osteoclast activity. Bone 62:99–107
Ross CL, Siriwardane M, Almeida-Porada G, Porada CD, Brink P, Christ GJ et al (2015) The effect of low-frequency electromagnetic field on human bone marrow stem/progenitor cell differentiation. Stem Cell Res 15:96–108
Bassett CA, Mitchell SN, Gaston SR (1982) Pulsing electromagnetic field treatment in ununited fractures and failed arthrodeses. JAMA 247:623–628
Simmons JW (1985) Treatment of failed posterior lumbar interbody fusion (PLIF) of the spine with pulsing electromagnetic fields. Clin Orthop Relat Res 193:127–132
Veronesi F, Torricelli P, Giavaresi G, Sartori M, Cavani F, Setti S et al (2014) In vivo effect of two different pulsed electromagnetic field frequencies on osteoarthritis. J Orthop Res 32:677–685
Bagnato GL, Miceli G, Marino N, Sciortino D, Bagnato GF (2016) Pulsed electromagnetic fields in knee osteoarthritis: a double blind, placebo-controlled, randomized clinical trial. Rheumatology 55:755–762
Yan JL, Zhou J, Ma HP, Ma XN, Gao YH, Shi WG et al (2015) Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia. Mol Cell Endocrinol 404:132–140
Xie YF, Shi WG, Zhou J, Gao YH, Li SF, Fang QQ et al (2016) Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRII localized at the base of primary cilium. Bone 93:22–32
Ma HP, Ming LG, Ge BF, Zhai YK, Song P, Xian CJ et al (2011) Icarrin is more potent than genistein in promoting osteoblast differentiation and mineralization in vitro. J Cell Biochem 112:916–923
Ma XN, Zhou J, Ge BF, Zhen P, Ma HP, Shi WG et al (2013) Icariin induces osteoblast differentiation and mineralization without dexamethasone in vitro. Planta Med 79:1501–1508
Janas A, Folwarczna J (2017) Opioid receptor agonists may favorably affect bone mechanical properties in rats with estrogen deficiency-induced osteoporosis. Naunyn Schmiedebergs Arch Pharmacol 390:175–185
Jeremy B, Adams EL, Beth B, Oleksra M, Czymmek KJ, Anja N (2012) Initiation of BMP2 signaling in domains on the plasma membrane. J Cell Physiol 227:2880–2888
Mardon J, Mathey J, Kati-Coulibaly S, Puel C, Davicco MJ, Lebecque P (2008) Influence of lifelong soy isoflavones consumption on bone mass in the rat. Exp Biol Med (Maywood) 233:229–237
Cummings SR, Black DM, Nevitt MC, Browner W, Cauley J, Ensrud K (1993) Bone density at various sites for prediction of hip fractures. The study of osteoporotic fractures research group. Lancet 34:72–75
Bonjour JP, Chevalley T, Ferrari S, Rizzoli R (2009) The importance and relevance of peak bone mass in the prevalence of osteoporosis. Salud Públ De Mexico 51:(Suppl 1):S5
Gautam AK, Bhargavan B, Tyagi AM, Srivastava K, Yadav DK, Kumar M et al (2011) Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J Nutr Biochem 22:318–327
Golden NH, Abrams SA (2014) Optimizing bone health in children and adolescents. Pediatrics 134:1229–1243
Viljakainen HT (2016) Factors influencing bone mass accrual: focus on nutritional aspects. Proc Nutr Soc 75:415–419
Weaver CM, Gordon CM, Janz KF, Kalkwarf HJ, Lappe JM, Lewis R (2016) The national osteoporosis foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 27:1281–1286
Funk RH, Monsees T, Ozkucur N (2009) Electromagnetic effects-from cell biology to medicine. Prog Histochem Cytochem 43:177–264
Zhou J, Ma XN, Gao YH, Yan JL, Shi WG, Xian CJ et al (2014) Different electromagnetic field waveforms have different effects on proliferation, differentiation and mineralization of osteoblasts in vitro. Bioelectromagnetics 35:30–40
Guicheux J, Lemonnier J, Ghayor C, Suzuki A, Palmer G, Caverzasio J (2003) Activation of p38 mitogen-activated protein kinase and c-Jun-NH2-terminal kinase by BMP-2 and their implication in the stimulation of osteoblastic cell differentiation. J Bone Miner Res 18:2060–2068
Fuentealba LC, Eivers E, Ikeda A, Hurtado C, Kuroda H, Pera EM et al (2007) Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 131:980–993
Rahman MS, Akhtar N, Jamil HM, Banik RS, Asaduzzaman SM (2015) TGF-β/BMP signaling and other molecular events: regulation of osteoblastogenesis and bone formation. Bone Res 3:15005
Zhao QR, Lu JM, Yao JJ, Zhang ZY, Ling C, Mei YA (2015) Neuritin reverses deficits in murine novel object associative recognition memory caused by exposure to extremely low-frequency (50 Hz) electromagnetic fields. Sci Rep 5:11768
Gurfinkel YI, At’kov OY, Vasin AL, Breus TK, Sasonko ML, Pishchalnikov RY (2016) Effect of zero magnetic field on cardiovascular system and microcirculation. Life Sci Space Res 8:1–7
Sun J, Kwan RL, Zheng Y, Cheing GL (2016) Effects of pulsed electromagnetic fields on peripheral blood circulation in people with diabetes: a randomized controlled trial. Bioelectromagnetics 37:290–297
Guerriero F, Ricevuti G (2016) Extremely low frequency electromagnetic fields stimulation modulates autoimmunity and immune responses: a possible immuno-modulatory therapeutic effect in neurodegenerative diseases. Neural Regen Res 11:1888–1895
Markov MS (2005) “Biological windows”: a tribute to W. Ross Adey. Environmentalist 25:67–74
Markov MS (2007) Magnetic field therapy: a review. Electromagn Biol Med 26:1–23
Acknowledgements
This work was supported by the National Natural Sciences Foundation of China (No. 81270963, 81471090, 81770879 to KMC, and 21762027 to ZDY) and the International Science & Technology Cooperation Program of China (No.2015DFR30940). CJX is supported by NHMRC Australia Senior Research Fellowship (No. 1042105).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Bao-Ying Zhu, Zhong-Duo Yang, Xin-Ru Chen, Jian Zhou, Yu-Hai Gao, Cory J Xian, and Ke-Ming Chen declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All animal experiments were carried out in accordance with the Guide for Use and Care of Laboratory Animals and were approved by the Lanzhou General Hospital of CPLA’s Animal Care Committee. This article does not contain any studies with human participants performed by any of the authors.
Rights and permissions
About this article
Cite this article
Zhu, B.Y., Yang, Z.D., Chen, X.R. et al. Exposure Duration Is a Determinant of the Effect of Sinusoidal Electromagnetic Fields on Peak Bone Mass of Young Rats. Calcif Tissue Int 103, 95–106 (2018). https://doi.org/10.1007/s00223-018-0396-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0396-2