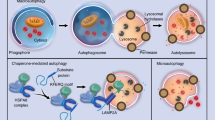
Abstract
Imbalances between bone formation and resorption are the primary cause of osteoporosis. However, currently, a detailed molecular mechanism of osteoporosis is not available. Autophagy is the conserved process characterized by degrading and recycling aggregated proteins, intracellular pathogens, and damaged organelles. MicroRNAs (miRNAs) are novel regulatory factors that play important roles in numerous cellular processes, including autophagy, through the posttranscriptional regulation of gene expression. Conversely, autophagy plays a role in the regulation of miRNA homeostasis. Recent advances have revealed that both autophagy and miRNAs are involved in the maintenance of bone homoeostasis, whereas the role of the interaction of miRNAs with autophagy in osteoporosis remains unclear. In this paper, we review previous reports on autophagy, miRNAs, and their interaction in osteoporosis.




Similar content being viewed by others
References
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795
Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR (2009) Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 301:513–521
van Staa TP, Dennison EM, Leufkens HG, Cooper C (2001) Epidemiology of fractures in England and Wales. Bone 29:517–522
Pasco JA, Lane SE, Brennan-Olsen SL, Holloway KL, Timney EN, Bucki-Smith G, Morse AG, Dobbins AG, Williams LJ, Hyde NK, Kotowicz MA (2015) The epidemiology of incident fracture from cradle to senescence. Calcif Tissue Int 97:568–576
Wolski H, Bogacz A, Bartkowiak-Wieczorek J, Greber A, Pieńkowski W, Drews K, Klejewski A, Seremak-Mrozikiewicz A (2015) Polymorphism of bone morphogenetic protein (BMP2) and osteoporosis etiology. Ginekol Pol 86:203–209
Maiuri MC, Zalckvar E, Kimchi A, Kroemer G (2007) Self-eating and self-killing: crosstalk between autophagy and apoptosis. Nat Rev Mol Cell Biol 8:741–752
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Levine B, Kroemer G (2008) Autophagy in the pathogenesis of disease. Cell 132:27–42
Levine B, Kroemer G (2009) Autophagy in aging, disease and death: the true identity of a cell death impostor. Cell Death Differ 16:1–2
Kim KH, Lee MS (2014) Autophagy—a key player in cellular and body metabolism. Nat Rev Endocrinol 10:322–337
Kim VN (2005) Small RNAs: classification, biogenesis, and function. Mol Cells 19:1–15
Landgraf P, Rusu M, Sheridan R et al (2007) A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 129:1401–1414
Kim VN, Han J, Siomi MC (2009) Biogenesis of small RNAs in animals. Nat Rev Mol Cell Biol 10:126–139
Meltzer PS (2005) Cancer genomics: small RNAs with big impacts. Nature 435:745–746
Lee RC, Feinbaum RL, Ambros V (1993) The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75:843–854
Friedman RC, Farh KK, Burge CB, Bartel DP (2009) Most mammalian mRNAs are conserved targets of microRNAs. Genome Res 19:92–105
Alvarez-Garcia I, Miska EA (2005) MicroRNA functions in animal development and human disease. Development 132:4653–4662
Kloosterman WP, Plasterk RH (2006) The diverse functions of microRNAs in animal development and disease. Dev Cell 11:441–450
Gibbings D, Mostowy S, Jay F, Schwab Y, Cossart P, Voinnet O (2012) Selective autophagy degrades DICER and AGO2 and regulates miRNA activity. Nat Cell Biol 14:1314–1321
Lan SH, Wu SY, Zuchini R, Lin XZ, Su IJ, Tsai TF, Lin YJ, Wu CT, Liu HS (2014) Autophagy suppresses tumorigenesis of hepatitis B virus-associated hepatocellular carcinoma through degradation of microRNA-224. Hepatology 59:505–517
Jing Z, Han W, Sui X, Xie J, Pan H (2015) Interaction of autophagy with microRNAs and their potential therapeutic implications in human cancers. Cancer Lett 356:332–338
Jin S (2006) Autophagy, mitochondrial quality control, and oncogenesis. Autophagy 2:80–84
Guertin DA, Sabatini DM (2007) Defining the role of mTOR in cancer. Cancer Cell 12:9–22
Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, Oroz LG, Scaravilli F, Easton DF, Duden R, O’Kane CJ, Rubinsztein DC (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585–595
Daroszewska A, van’t Hof RJ, Rojas JA, Layfield R, Landao-Basonga E, Rose L, Rose K, Ralston SH (2011) A point mutation in the ubiquitin-associated domain of SQSMT1 is sufficient to cause a Paget’s disease-like disorder in mice. Hum Mol Genet 20:2734–2744
Shapiro IM, Layfield R, Lotz M, Settembre C, Whitehouse C (2014) Boning up on autophagy: the role of autophagy in skeletal biology. Autophagy 10:7–19
Romero DF, Buchinsky FJ, Rucinski B, Cvetkovic M, Bryer HP, Liang XG, Ma YF, Jee WS, Epstein S (1995) Rapamycin: a bone sparing immunosuppressant? J Bone Miner Res 10:760–768
Alvarez-Garcia O, Carbajo-Pérez E, Garcia E, Gil H, Molinos I, Rodriguez J, Ordoñez FA, Santos F (2007) Rapamycin retards growth and causes marked alterations in the growth plate of young rats. Pediatr Nephrol 22:954–961
Sanchez CP, He YZ (2009) Bone growth during rapamycin therapy in young rats. BMC Pediatr 9:3
Hussein O, Tiedemann K, Murshed M, Komarova SV (2012) Rapamycin inhibits osteolysis and improves survival in a model of experimental bone metastases. Cancer Lett 314:176–184
Alvarez-García O, García-López E, Loredo V, Gil-Peña H, Rodríguez-Suárez J, Ordóñez FA, Carbajo-Pérez E, Santos F (2010) Rapamycin induces growth retardation by disrupting angiogenesis in the growth plate. Kidney Int 78:561–568
Holstein JH, Klein M, Garcia P, Histing T, Culemann U, Pizanis A, Laschke MW, Scheuer C, Meier C, Schorr H, Pohlemann T, Menger MD (2008) Rapamycin affects early fracture healing in mice. Br J Pharmacol 154:1055–1062
Huang B, Wang Y, Wang W, Chen J, Lai P, Liu Z, Yan B, Xu S, Zhang Z, Zeng C, Rong L, Liu B, Cai D, Jin D, Bai X (2015) MTORC1 prevents preosteoblast differentiation through the Notch signaling pathway. PLoS Genet 11:e1005426
Darcy A, Meltzer M, Miller J, Lee S, Chappell S, Ver Donck K, Montano M (2012) A novel library screen identifies immunosuppressors that promote osteoblast differentiation. Bone 50:1294–1303
Ogawa T, Tokuda M, Tomizawa K, Matsui H, Itano T, Konishi R, Nagahata S, Hatase O (1998) Osteoblastic differentiation is enhanced by rapamycin in rat osteoblast-like osteosarcoma (ROS 17/2.8) cells. Biochem Biophys Res Commun 249:226–230
Suvannasankha A, Chirgwin JM (2014) Role of bone-anabolic agents in the treatment of breast cancer bone metastases. Breast Cancer Res 16:484
Isomoto S, Hattori K, Ohgushi H, Nakajima H, Tanaka Y, Takakura Y (2007) Rapamycin as an inhibitor of osteogenic differentiation in bone marrow-derived mesenchymal stem cells. J Orthop Sci 12:83–88
Hirose K, Shiomi T, Hozumi S, Kikuchi Y (2014) Mechanistic target of rapamycin complex 1 signaling regulates cell proliferation, cell survival, and differentiation in regenerating zebrafish fins. BMC Dev Biol 14:42
Singha UK, Jiang Y, Yu S, Luo M, Lu Y, Zhang J, Xiao G (2008) Rapamycin inhibits osteoblast proliferation and differentiation in MC3T3-E1 cells and primary mouse bone marrow stromal cells. J Cell Biochem 103:434–446
Yeh LC, Ma X, Ford JJ, Adamo ML, Lee JC (2013) Rapamycin inhibits BMP-7-induced osteogenic and lipogenic marker expressions in fetal rat calvarial cells. J Cell Biochem 114:1760–1771
Kim J, Jung Y, Sun H, Joseph J, Mishra A, Shiozawa Y, Wang J, Krebsbach PH, Taichman RS (2012) Erythropoietin mediated bone formation is regulated by mTOR signaling. J Cell Biochem 113:220–228
Li SF, Tang JJ, Chen J, Zhang P, Wang T, Chen TY, Yan B, Huang B, Wang L, Huang MJ, Zhang ZM, Jin DD (2015) Regulation of bone formation by baicalein via the mTORC1 pathway. Drug Des Dev Ther 9:5169–5183
Sun H, Kim JK, Mortensen R, Mutyaba LP, Hankenson KD, Krebsbach PH (2013) Osteoblast-targeted suppression of PPARgamma increases osteogenesis through activation of mTOR signaling. Stem Cells 31:2183–2192
Fan JB, Liu W, Zhu XH, Yuan K, Xu DW, Chen JJ, Cui ZM (2015) EGFR-AKT-mTOR activation mediates epiregulin-induced pleiotropic functions in cultured osteoblasts. Mol Cell Biochem 398:105–113
Lisse TS, Liu T, Irmler M, Beckers J, Chen H, Adams JS, Hewison M (2011) Gene targeting by the vitamin D response element binding protein reveals a role for vitamin D in osteoblast mTOR signaling. FASEB J 25:937–947
Tchetina EV, Maslova KA, Krylov MY, Myakotkin VA (2015) Association of bone loss with the upregulation of survival-related genes and concomitant downregulation of Mammalian target of rapamycin and osteoblast differentiation-related genes in the peripheral blood of late postmenopausal osteoporotic women. J Osteoporos 2015:802694
Fang F, Sun S, Wang L, Guan JL, Giovannini M, Zhu Y, Liu F (2015) Neural crest-specific TSC1 deletion in mice leads to sclerotic craniofacial bone lesion. J Bone Miner Res 30:1195–1205
Zeng Z, Jing D, Zhang X, Duan Y, Xue F (2015) Cyclic mechanical stretch promotes energy metabolism in osteoblast-like cells through an mTOR signaling-associated mechanism. Int J Mol Med 36:947–956
Riddle RC, Leslie JM, Gross TS, Clemens TL (2011) Hypoxia-inducible factor-1alpha protein negatively regulates load-induced bone formation. J Biol Chem 286:44449–44456
Luo D, Ren H, Li T, Lian K, Lin D (2015) Rapamycin reduces severity of senile osteoporosis by activating osteocyte autophagy. Osteoporos Int. doi:10.1007/s00198-015-3325-5
van der Merwe SW, Conradie MM, Bond R, Olivier BJ, Fritz E, Nieuwoudt M, Delport R, Slavik T, Engelbrecht G, Kahn D, Shephard EG, Kotze MJ, de Villiers NP, Hough S (2006) Effect of rapamycin on hepatic osteodystrophy in rats with portasystemic shunting. World J Gastroenterol 12:4504–4510
Liu N, Xu N, Wei LH, Chai GL (2013) Mammalian target of rapamycin inhibitor abrogates abnormal osteoclastogenesis in neurofibromatosis type 1. Chin Med J (Engl) 126:101–107
Kloos B, Chakraborty S, Lindner SG, Noack K, Harre U, Schett G, Krämer OH, Kubatzky KF (2015) Pasteurella multocida toxin-induced osteoclastogenesis requires mTOR activation. Cell Commun Signal 13:40
Francis LK, Alsayed Y, Leleu X, Jia X, Singha UK, Anderson J, Timm M, Ngo H, Lu G, Huston A, Ehrlich LA, Dimmock E, Lentzsch S, Hideshima T, Roodman GD, Anderson KC, Ghobrial IM (2006) Combination mammalian target of rapamycin inhibitor rapamycin and HSP90 inhibitor 17-allylamino-17-demethoxygeldanamycin has synergistic activity in multiple myeloma. Clin Cancer Res 12:6826–6835
Indo Y, Takeshita S, Ishii KA, Hoshii T, Aburatani H, Hirao A, Ikeda K (2013) Metabolic regulation of osteoclast differentiation and function. J Bone Miner Res 28:2392–2399
Owen HC, Vanhees I, Gunst J, Van Cromphaut S, Van den Berghe G (2015) Critical illness-induced bone loss is related to deficient autophagy and histone hypomethylation. Intensive Care Med Exp 3:52
Smink JJ, Tunn PU, Leutz A (2012) Rapamycin inhibits osteoclast formation in giant cell tumor of bone through the C/EBPbeta-MafB axis. J Mol Med (Berl) 90:25–30
Blaslov K, Katalinic L, Kes P, Spasovski G, Smalcelj R, Basic-Jukic N (2014) What is the impact of immunosuppressive treatment on the post-transplant renal osteopathy? Int Urol Nephrol 46:1019–1024
Cejka D, Hayer S, Niederreiter B, Sieghart W, Fuereder T, Zwerina J, Schett G (2010) Mammalian target of rapamycin signaling is crucial for joint destruction in experimental arthritis and is activated in osteoclasts from patients with rheumatoid arthritis. Arthritis Rheum 62:2294–2302
Sugatani T, Hruska KA (2005) Akt1/Akt2 and mammalian target of rapamycin/Bim play critical roles in osteoclast differentiation and survival, respectively, whereas Akt is dispensable for cell survival in isolated osteoclast precursors. J Biol Chem 280:3583–3589
Glantschnig H, Fisher JE, Wesolowski G, Rodan GA, Reszka AA (2003) M-CSF, TNFalpha and RANK ligand promote osteoclast survival by signaling through mTOR/S6 kinase. Cell Death Differ 10:1165–1177
Hadji P, Coleman R, Gnant M (2013) Bone effects of mammalian target of rapamycin (mTOR) inhibition with everolimus. Crit Rev Oncol Hematol 87:101–111
Durán A, Serrano M, Leitges M, Flores JM, Picard S, Brown JP, Moscat J, Diaz-Meco MT (2004) The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell 6:303–309
Li H, Xie H, Liu W, Hu R, Huang B, Tan YF, Xu K, Sheng ZF, Zhou HD, Wu XP, Luo XH (2009) A novel microRNA targeting HDAC5 regulates osteoblast differentiation in mice and contributes to primary osteoporosis in humans. J Clin Invest 119:3666–3677
Wang Y, Li L, Moore BT, Peng XH, Fang X, Lappe JM, Recker RR, Xiao P (2012) MiR-133a in human circulating monocytes: a potential biomarker associated with postmenopausal osteoporosis. PLoS One 7:e34641
Wang X, Guo B, Li Q et al (2013) MiR-214 targets ATF4 to inhibit bone formation. Nat Med 19:93–100
Lei SF, Papasian CJ, Deng HW (2011) Polymorphisms in predicted miRNA binding sites and osteoporosis. J Bone Miner Res 26:72–78
Seeliger C, Karpinski K, Haug AT, Vester H, Schmitt A, Bauer JS, van Griensven M (2014) Five freely circulating miRNAs and bone tissue miRNAs are associated with osteoporotic fractures. J Bone Miner Res 29:1718–1728
Bae Y, Yang T, Zeng HC, Campeau PM, Chen Y, Bertin T, Dawson BC, Munivez E, Tao J, Lee BH (2012) miRNA-34c regulates Notch signaling during bone development. Hum Mol Genet 21:2991–3000
Yang N, Wang G, Hu C, Shi Y, Liao L, Shi S, Cai Y, Cheng S, Wang X, Liu Y, Tang L, Ding Y, Jin Y (2013) Tumor necrosis factor alpha suppresses the mesenchymal stem cell osteogenesis promoter miR-21 in estrogen deficiency-induced osteoporosis. J Bone Miner Res 28:559–573
Ell B, Mercatali L, Ibrahim T, Campbell N, Schwarzenbach H, Pantel K, Amadori D, Kang Y (2013) Tumor-induced osteoclast miRNA changes as regulators and biomarkers of osteolytic bone metastasis. Cancer Cell 24:542–556
Krishnan V, Bryant HU, Macdougald OA (2006) Regulation of bone mass by Wnt signaling. J Clin Invest 116:1202–1209
Baron R, Kneissel M (2013) WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med 19:179–192
Qiu W, Kassem M (2014) MiR-141-3p inhibits human stromal (mesenchymal) stem cell proliferation and differentiation. Biochim Biophys Acta 1843:2114–2121
Chen Q, Liu W, Sinha KM, Yasuda H, de Crombrugghe B (2013) Identification and characterization of microRNAs controlled by the osteoblast-specific transcription factor Osterix. PLoS One 8:e58104
Kapinas K, Kessler CB, Delany AM (2009) MiR-29 suppression of osteonectin in osteoblasts: regulation during differentiation and by canonical Wnt signaling. J Cell Biochem 108:216–224
Li Z, Hassan MQ, Jafferji M, Aqeilan RI, Garzon R, Croce CM, van Wijnen AJ, Stein JL, Stein GS, Lian JB (2009) Biological functions of miR-29b contribute to positive regulation of osteoblast differentiation. J Biol Chem 284:15676–15684
Kapinas K, Kessler C, Ricks T, Gronowicz G, Delany AM (2010) MiR-29 modulates Wnt signaling in human osteoblasts through a positive feedback loop. J Biol Chem 285:25221–25231
Wang T, Xu Z (2010) MiR-27 promotes osteoblast differentiation by modulating Wnt signaling. Biochem Biophys Res Commun 402:186–189
Hu W, Ye Y, Zhang W, Wang J, Chen A, Guo F (2013) MiR1423p promotes osteoblast differentiation by modulating Wnt signaling. Mol Med Rep 7:689–693
Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J (2011) Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Miner Res 26:1953–1963
Zhang WB, Zhong WJ, Wang L (2014) A signal-amplification circuit between miR-218 and Wnt/beta-catenin signal promotes human adipose tissue-derived stem cells osteogenic differentiation. Bone 58:59–66
Wang FS, Chuang PC, Lin CL, Chen MW, Ke HJ, Chang YH, Chen YS, Wu SL, Ko JY (2013) MicroRNA-29a protects against glucocorticoid-induced bone loss and fragility in rats by orchestrating bone acquisition and resorption. Arthritis Rheum 65:1530–1540
Shi C, Huang P, Kang H, Hu B, Qi J, Jiang M, Zhou H, Guo L, Deng L (2015) Glucocorticoid inhibits osteoblasts proliferation by microRNA-199a targeting WNT signaling. J Mol Endocrinol 54:325–337
Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP (2014) Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One 9:e114627
Kim YJ, Bae SW, Yu SS, Bae YC, Jung JS (2009) MiR-196a regulates proliferation and osteogenic differentiation in mesenchymal stem cells derived from human adipose tissue. J Bone Miner Res 24:816–825
Mizuno Y, Tokuzawa Y, Ninomiya Y, Yagi K, Yatsuka-Kanesaki Y, Suda T, Fukuda T, Katagiri T, Kondoh Y, Amemiya T, Tashiro H, Okazaki Y (2009) MiR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett 583:2263–2268
Cheng P, Chen C, He HB, Hu R, Zhou HD, Xie H, Zhu W, Dai RC, Wu XP, Liao EY, Luo XH (2013) MiR-148a regulates osteoclastogenesis by targeting V-maf musculoaponeurotic fibrosarcoma oncogene homolog B. J Bone Miner Res 28:1180–1190
Kim K, Kim JH, Lee J, Jin HM, Kook H, Kim KK, Lee SY, Kim N (2007) MafB negatively regulates RANKL-mediated osteoclast differentiation. Blood 109:3253–3259
Sugatani T, Vacher J, Hruska KA (2011) A microRNA expression signature of osteoclastogenesis. Blood 117:3648–3657
Cao Z, Moore BT, Wang Y, Peng XH, Lappe JM, Recker RR, Xiao P (2014) MiR-422a as a potential cellular microRNA biomarker for postmenopausal osteoporosis. PLoS One 9:e97098
Guo LJ, Liao L, Yang L, Li Y, Jiang TJ (2014) MiR-125a TNF receptor-associated factor 6 to inhibit osteoclastogenesis. Exp Cell Res 321:142–152
Zhao C, Sun W, Zhang P, Ling S, Li Y, Zhao D, Peng J, Wang A, Li Q, Song J, Wang C, Xu X, Xu Z, Zhong G, Han B, Chang YZ, Li Y (2015) MiR-214 promotes osteoclastogenesis by targeting Pten/PI3k/Akt pathway. RNA Biol 12:343–353
Krzeszinski JY, Wei W, Huynh H, Jin Z, Wang X, Chang TC, Xie XJ, He L, Mangala LS, Lopez-Berestein G, Sood AK, Mendell JT, Wan Y (2014) MiR-34a blocks osteoporosis and bone metastasis by inhibiting osteoclastogenesis and Tgif2. Nature 512:431–435
Chen C, Cheng P, Xie H, Zhou HD, Wu XP, Liao EY, Luo XH (2014) MiR-503 regulates osteoclastogenesis via targeting RANK. J Bone Miner Res 29:338–347
Kagiya T, Nakamura S (2013) Expression profiling of microRNAs in RAW264.7 cells treated with a combination of tumor necrosis factor alpha and RANKL during osteoclast differentiation. J Periodontal Res 48:373–385
Rossi M, Pitari MR, Amodio N, Di Martino MT, Conforti F, Leone E, Botta C, Paolino FM, Del Giudice T, Iuliano E, Caraglia M, Ferrarini M, Giordano A, Tagliaferri P, Tassone P (2013) MiR-29b negatively regulates human osteoclastic cell differentiation and function: implications for the treatment of multiple myeloma-related bone disease. J Cell Physiol 228:1506–1515
Lee Y, Kim HJ, Park CK, Kim YG, Lee HJ, Kim JY, Kim HH (2013) MicroRNA-124 regulates osteoclast differentiation. Bone 56:383–389
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Gibbings D, Mostowy S, Voinnet O (2013) Autophagy selectively regulates miRNA homeostasis. Autophagy 9:781–783
Kim KM, Lim SK (2014) Role of miRNAs in bone and their potential as therapeutic targets. Curr Opin Pharmacol 16:133–141
Hocking LJ, Whitehouse C, Helfrich MH (2012) Autophagy: a new player in skeletal maintenance? J Bone Miner Res 27:1439–1447
Zhang GY, Wang J, Jia YJ, Han R, Li P, Zhu DN (2015) MicroRNA-9 promotes the neuronal differentiation of rat bone marrow mesenchymal stem cells by activating autophagy. Neural Regen Res 10:314–320
You L, Gu W, Chen L, Pan L, Chen J, Peng Y (2014) MiR-378 overexpression attenuates high glucose-suppressed osteogenic differentiation through targeting CASP3 and activating PI3K/Akt signaling pathway. Int J Clin Exp Pathol 7:7249–7261
Li H, Li T, Fan J, Li T, Fan L, Wang S, Weng X, Han Q, Zhao RC (2015) MiR-216a rescues dexamethasone suppression of osteogenesis, promotes osteoblast differentiation and enhances bone formation, by regulating c-Cbl-mediated PI3K/AKT pathway. Cell Death Differ 22:1935–1945
Weinbaum S, Cowin SC, Zeng Y (1994) A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 27:339–360
Kreke MR, Huckle WR, Goldstein AS (2005) Fluid flow stimulates expression of osteopontin and bone sialoprotein by bone marrow stromal cells in a temporally dependent manner. Bone 36:1047–1055
Mukai M, Yoshimine Y, Akamine A, Maeda K (1993) Bone-like nodules formed in vitro by rat periodontal ligament cells. Cell Tissue Res 271:453–460
Cho MI, Matsuda N, Lin WL, Moshier A, Ramakrishnan PR (1992) In vitro formation of mineralized nodules by periodontal ligament cells from the rat. Calcif Tissue Int 50:459–467
Qi L, Zhang Y (2014) The microRNA 132 regulates fluid shear stress-induced differentiation in periodontal ligament cells through mTOR signaling pathway. Cell Physiol Biochem 33:433–445
Yang M, Pan Y, Zhou Y (2014) MiR-96 promotes osteogenic differentiation by suppressing HBEGF-EGFR signaling in osteoblastic cells. FEBS Lett 588:4761–4768
Yu S, Geng Q, Ma J, Sun F, Yu Y, Pan Q, Hong A (2013) Heparin-binding EGF-like growth factor and miR-1192 exert opposite effect on Runx2-induced osteogenic differentiation. Cell Death Dis 4:e868
Sun KT, Chen MY, Tu MG, Wang IK, Chang SS, Li CY (2015) MicroRNA-20a regulates autophagy related protein-ATG16L1 in hypoxia-induced osteoclast differentiation. Bone 73:145–153
Jin Y, Tymen SD, Chen D, Fang ZJ, Zhao Y, Dragas D, Dai Y, Marucha PT, Zhou X (2013) MicroRNA-99 family targets AKT/mTOR signaling pathway in dermal wound healing. PLoS One 8:e64434
Wei F, Liu Y, Guo Y, Xiang A, Wang G, Xue X, Lu Z (2013) miR-99b-targeted mTOR induction contributes to irradiation resistance in pancreatic cancer. Mol Cancer 12:81
Franceschetti T, Dole NS, Kessler CB, Lee SK, Delany AM (2014) Pathway analysis of microRNA expression profile during murine osteoclastogenesis. PLoS One 9:e107262
M’Baya-Moutoula E, Louvet L, Metzinger-Le Meuth V, Massy ZA, Metzinger L (2015) High inorganic phosphate concentration inhibits osteoclastogenesis by modulating miR-223. Biochim Biophys Acta 1852:2202–2212
Yao Y, Jia T, Pan Y, Gou H, Li Y, Sun Y, Zhang R, Zhang K, Lin G, Xie J, Li J, Wang L (2015) Using a novel microRNA delivery system to inhibit osteoclastogenesis. Int J Mol Sci 16:8337–8350
Youngson NA, Lin PC, Lin SS (2014) The convergence of autophagy, small RNA and the stress response-implications for transgenerational epigenetic inheritance in plants. Biomol Concepts 5:1–8
Buchan JR (2014) mRNP granules. Assembly, function, and connections with disease. RNA Biol 11:1019–1030
Ling SC, Polymenidou M, Cleveland DW (2013) Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron 79:416–438
Deng H, Gao K, Jankovic J (2014) The role of FUS gene variants in neurodegenerative diseases. Nat Rev Neurol 10:337–348
Ryu HH, Jun MH, Min KJ, Jang DJ, Lee YS, Kim HK, Lee JA (2014) Autophagy regulates amyotrophic lateral sclerosis-linked fused in sarcoma-positive stress granules in neurons. Neurobiol Aging 35:2822–2831
Funding
This study is supported by Natural Science Foundation of China [81503591], the Science and Technology Projects of Guangdong Province [2014A020221021], the Natural Science Foundation of Guangdong Province [2014A030310082], a Grant from Guangzhou University of Chinese Medicine for excellent Young Scholars Project [KAB110133K04], and the Disciplinary Construction Fund of Education Department in Guangdong Province for Young Researcher Project [2013LYM-0012].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Gengyang Shen, Hui Ren, Ting Qiu, De Liang, Bo Xie, Zhida Zhang, Zhensong Yao, Zhidong Yang and Xiaobing Jiang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Rights and permissions
About this article
Cite this article
Shen, G., Ren, H., Qiu, T. et al. Implications of the Interaction Between miRNAs and Autophagy in Osteoporosis. Calcif Tissue Int 99, 1–12 (2016). https://doi.org/10.1007/s00223-016-0122-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0122-x