Abstract
Primary Sjögren’s syndrome (pSS) can be complicated by distal renal tubular acidosis (dRTA), which may contribute to low bone mineral density (BMD). Our objective was to evaluate BMD in pSS patients with and without dRTA as compared with healthy controls. BMD of lumbar spine (LS) and femoral neck (FN) was measured in 54 pSS patients and 162 healthy age- and sex-matched controls by dual-energy X-ray absorptiometry (DXA). dRTA was defined as inability to reach urinary pH <5.3 after an ammonium chloride (NH4Cl) test. LS- and FN-BMD were significantly higher in pSS patients compared with controls (1.18 ± 0.21 g/cm2 for patients vs. 1.10 ± 0.18 g/cm2 for controls, P = 0.008 and 0.9 ± 0.16 g/cm2 for patients vs. 0.85 ± 0.13 g/cm2 for controls, P = 0.009, respectively). After adjustment for BMI and smoking, the LS- and FN-BMD remained significantly higher. Patients with dRTA (N = 15) did not have a significantly different LS- and FN-BMD compared with those without dRTA (N = 39) after adjustment for BMI, age, and gender. Thirty-seven (69 %) pSS patients were using hydroxychloroquine (HCQ). Unexpectedly, pSS patients had a significantly higher LS- and FN-BMD compared with healthy controls. Patients with dRTA had similar BMD compared with patients without dRTA. We postulate that an explanation for the higher BMD in pSS patients may be the frequent use of HCQ.
Similar content being viewed by others
Introduction
Sjögren syndrome (SS) is a prevalent chronic autoimmune disease characterized by impairment of exocrine glands and systemic manifestations, affecting between 1 and 3 % of the general population [1]. SS can be present alone (primary Sjögren syndrome (pSS)) or accompanied by other autoimmune diseases such as systemic lupus erythematosus (SLE) or rheumatoid arthritis (RA) and is then called secondary Sjögren syndrome (sSS) [2, 3]. The classical symptoms of SS include dryness of mouth (xerostomia), dryness of eyes (xerophthalmia) and less commonly dryness of pharynx, larynx, and/or vagina [4]. Extraglandular manifestations can be divided into general symptoms (e.g., fatigue, arthralgia, and myalgia) and in systemic manifestations [5]. Common systemic manifestations include renal tubular acidosis, non-erosive symmetrical arthritis, interstitial lung disease, peripheral polyneuropathy, autoimmune thyroiditis, and B cell lymphoma [6–11].
Distal renal tubular acidosis (dRTA) is one of the less well recognized complications. Recently, we reported a considerably high prevalence of dRTA in pSS [7]. dRTA is characterized by the inability of patients to lower urinary pH <5.3 due to a defect in the proton secreting machinery of the alpha-intercalated cells in the collecting ducts of the kidney [12]. Patients with dRTA have a non-anion gap metabolic acidosis with urinary pH ≥5.3.
The association between chronic metabolic acidosis and alteration in bone cell function has been demonstrated both in vitro and in vivo [13, 14]. During metabolic acidosis, there appears to be an exchange of protons for calcium ions in bone mineral to buffer the excess of protons [13]. The metabolic effects of dRTA in pSS remain conflicting. Several case-series have shown a low bone mineral density (BMD) in patients with dRTA in pSS [15, 16]. Some recent studies report an increased prevalence of low BMD in patients with dRTA [17, 18], while other studies did not report a significant difference in patients with dRTA [19, 20]. Epidemiologic data on BMD in SS are lacking.
We hypothesized that BMD is significantly decreased in patients with pSS and especially in those with dRTA. Therefore, our aim was to evaluate BMD in pSS patients with and without dRTA as compared with healthy controls.
Methods
Study Cohort
Patients were selected from the outpatient clinic of the department of internal medicine (division of clinical immunology) of Erasmus MC in Rotterdam, The Netherlands. pSS was defined according to the Revised American-European classification criteria [21]. The results of salivary gland biopsy were retrieved when available. Additional inclusion criteria for this study included age >18 years and an estimated glomerular filtration rate >30 ml/min. The exclusion criteria were other underlying autoimmune diseases, known risk factors for osteoporosis (vitamin D level <20 nmol/l, untreated hyperthyroidism, hyperparathyroidism, use of corticosteroids (prednisone equivalent of >7.5 mg for >3 months in the last year), use of bisphosphonates, multiple myeloma, mastocytosis). All participants were asked about menopausal status (if applicable), current smoking and history of fractures, and use of medication (Table 1). Data from BMD in the healthy control group were obtained from the ERF (Erasmus Rucphen Family) study database [22]. The matching criteria for the controls were age and sex. For every pSS patient, three controls were selected.
Bone Mineral Density
In all subjects, we measured BMD of the lumbar spine (L2-L4) and femoral neck using a dual-energy X-ray absorptiometry (DXA) scanner (Prodigy Pro Full P8, enCORE™ Software Platform, GE Medical Systems Lunar, Belgium). Scans were performed according to the manufacturer’s guidelines and analyzed according to ISCD rules [23]. The healthy control group was scanned with a different DXA device from the same type (GE Lunar Prodigy device, GE Healthcare, USA) [24]. As described by Enneman et al., a cross-calibration was performed using a spine phantom which showed that the measurements of the new scanner (the one we used for the patients in the current study) were slightly higher by a factor 1.0101 [24]. Therefore, we divided our results by this factor for comparison with the data from the ERF study. BMD was expressed in grams per square centimeters.
Biochemical Parameters
In all patients, we measured vitamin D status. Anti-nuclear antibodies (ANA), SSA/Ro52, SSA/Ro60, SSB/La auto-antibodies, and rheumatoid factor (RF) were also measured in all patients using previously reported methods [25]. Serum was collected before 10:00 a.m. and analyzed the same day. Patients were not instructed to be fasting. The following bone turnover markers (BTMs) were measured in patients: serum N-terminal propeptide of type I procollagen (PINP) and serum bone-specific alkaline phosphatase (BAP, both as measures of bone formation) and serum N-terminal crosslinking telopeptide of type I collagen (NTX, as measure for bone resorption). There were no data available on BTMs in the healthy control group.
Distal Renal Tubular Acidosis
dRTA was defined as an abnormal NH4CL test and the absence of any other known causes for dRTA (e.g., medication, hypercalciuria) [12]. The NH4CL test is defined as abnormal if patients fail to achieve a urinary pH <5.3 within 4 h after intake of ammonium chloride (1 ml/kg body weight) [26].
Statistics
All results are expressed as means with standard deviations. Comparisons of the normally distributed continuous variables between two groups were performed using the student T test. Since BTMs were not normally distributed, we compared BTMs between the two groups using the Mann–Whitney U test. Linear regression analysis was used to estimate the effect of having pSS on BMD before and after adjustment for body mass index (BMI) and smoking. Linear regression analysis was used to estimate the effect of having dRTA on BMD before and after adjustment for BMI, age, and gender. A P value <0.05 was considered significant. All analyses were performed in SPSS (version 21, IBM).
Results
Study Cohort and Baseline Characteristics
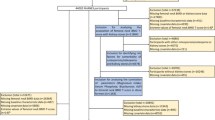
The study cohort included 54 patients with pSS and the control group consisted of 162 subjects. Initially, 62 patients participated in the study. Eight patients were excluded, including four patients who were unable to complete the NH4CL test due to repeated vomiting, one patient in whom the DXA-scan was not reliable due to scoliosis and three patients were using bisphosphonates. No patients were excluded because of long-term use of corticosteroids and only three patients were using low dose corticosteroids for a medical condition other than pSS. The baseline characteristics of the study cohort are shown in (Table 1). Similar to previous studies on pSS, our cohort has a female:male ratio of approximately 10:1 [27]. Thirty-seven (69 %) patients with pSS were using HCQ. In addition to medication for pSS, other commonly used drugs in this cohort were vitamin D with calcium supplements (N = 11). None of these patients reported a fracture in their medical history.
BMD of pSS Patients Compared with Healthy Controls
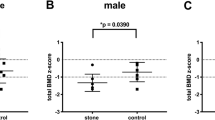
BMD of fifty-four pSS patients was compared with the age- and sex-matched control group of 162 subjects. The LS- and FN-BMD were significantly higher in the pSS patients compared with the healthy control group (1.18 ± 0.21 g/cm2 for pSS patients vs. 1.10 ± 0.18 g/cm2 for the control group, P = 0.008 and 0.9 ± 0.16 g/cm2 for pSS patients vs. 0.85 ± 0.13 g/cm2 for the control group, P = 0.009, respectively) (Fig. 1 ).
Comparison of BMD between patients and controls. The graph shows the bone mineral density of the lumbar spine and the femoral neck of patients with primary Sjögren syndrome (N = 54) compared with healthy age- and sex-matched controls (N = 162). SD standard deviation, BMD bone mineral density, LS-BMD bone mineral density of the lumbar spine, FN-BMD bone mineral density of the femoral neck
After adjustment for BMI and smoking, the LS- and FN-BMD remained significantly higher (β = 0.10 ± 0.030 g/cm2, P < 0.001 and β = 0.077 ± 0.022 g/cm2, P < 0.001) (Table 2 ).
The Effect of Distal Renal Tubular Acidosis on BMD
Fifteen pSS patients had an urinary acidification defect as measured by the NH4CL test. Between both groups the levels of biochemical parameters and the use of medication were similar (Table 1 ). Patients with dRTA were significantly younger compared with those without dRTA (Table 1 ). Both the LS- and FN-BMD were significantly higher in patients with an urinary acidification defect compared with those without an urinary acidification defect (LS: 1.29 ± 0.16 g/cm2 vs. 1.14 ± 0.21 g/cm2, P = 0.018 and FN: 1.0 ± 0.19 g/cm2 vs. 0.87 ± 0.14 g/cm2, P = 0.007). After adjustment for BMI, age, and gender, both the LS- and FN-BMD were not significantly higher anymore (LS: β = 0.12 ± 0.064 g/cm2, P = 0.065 and FN: β = 0.07 ± 0.049 g/cm2, P = 0.16) (Table 3 ).
Bone Turnover Markers in pSS Patients
In patients with dRTA serum, PINP was not significantly higher compared with patients without dRTA (P = 0.093). The other marker for bone formation, BAP, was also not significantly different between both groups (P = 0.11). The bone resorption marker NTX was not significantly different between patients with and without dRTA (P = 0.92) (data not shown).
Discussion
In the present study, we found that, contrary to expected, pSS patients have significantly higher BMD than healthy age- and sex-matched controls. We searched the available literature but did not find another study reporting BMD measurements in pSS patients as compared with a healthy control group. Studies concerning BMD in autoimmune diseases are mainly performed in lupus patients. In agreement with Arampatzis et al. and Pongchaiyakul et al., we found that patients with an urinary acidification defect did not have a significantly different LS- and FN-BMD compared with those patients without an urinary acidification defect [19, 20].
Bushinsky et al. reported a decreased bone mineralization in an acidotic environment in both in vitro and in vivo studies [13, 14]. This makes us wonder what the reason is that we did not find a lower BMD in patients with dRTA.
We hypothesize that the observed BMD in pSS patients may be related to the use of hydroxychloroquine (HCQ) which the majority (69 %) of patients in our study was using. In case of systemic manifestations, therapy with non-steroidal anti-inflammatory drugs or HCQ is advised. HCQ has proven to be effective against fatigue, arthralgia, and myalgia [28, 29]. Lakshminarayanan et al. and Mok et al. reported that in lupus the use of HCQ was associated with increased BMD of the hip [30, 31]. In both studies, disease activity and use of corticosteroids were not significantly different between both groups. Additionally, Xiu et al. recently reported a reduced osteoclastogenesis by TRAF3 degradation due to the effects of chloroquine in mice, which may suggest that HCQ has direct effects on bone metabolism [32]. Based on these clinical and biochemical studies, we hypothesize that HCQ may have beneficial effects on BMD.
In our cohort, it is unknown how long these patients were treated with HCQ. We also did not have information about past use of HCQ in patients, who are not using it currently. Therefore, analyzing a possible association between HCQ use and BMD would not be reliable in our cohort. To demonstrate whether the use of HCQ has beneficial effects on human bone cells, in vitro studies should be performed.
We analyzed whether patients with dRTA also had different BTM measurements compared with patients without an urinary acidification defect. Since patients with dRTA had similar LS- and FN-BMD compared with those without dRTA, we expected that the BTMs measurements would not be significantly different between both groups. Indeed, all three BTMs (PINP, NTX, and BAP) were not significantly different between patients with and without an urinary acidification defect. Unfortunately, we could not compare BTM measurements between pSS patients and the healthy control group since data about BTM measurements in the healthy controls is lacking.
The strength of this study is that we have reported new data about the BMD values in a large cohort of pSS patients. In addition, we analyzed the effects of dRTA, a common complication of pSS, on BMD in pSS patients. A limitation of this study is that we used a different DXA scanner compared to Zillikens et al. although the type of machine was the same and calibration was performed with a spine phantom, making this an unlikely explanation for our findings [22].
In conclusion, we found that both the LS- and FN-BMD were higher in patients with pSS than in age and sex-matched healthy controls. In addition, LS- and FN-BMD in patients with an urinary acidification defect is comparable with patients without an urinary acidification defect. An explanation for the high BMD in pSS patients may be the frequent use of HCQ, but future studies will have to confirm whether indeed use of HCQ is associated with higher BMD.
References
Peri Y, Agmon-Levin N, Theodor E, Shoenfeld Y (2012) Sjogren’s syndrome, the old and the new. Best Pract Res Clin Rheumatol 26(1):105–117. doi:10.1016/j.berh.2012.01.012
Baer AN, Maynard JW, Shaikh F, Magder LS, Petri M (2010) Secondary Sjogren’s syndrome in systemic lupus erythematosus defines a distinct disease subset. J Rheumatol 37(6):1143–1149. doi:10.3899/jrheum.090804
Hage MP, Al-Badri MR, Azar ST (2014) A favorable effect of hydroxychloroquine on glucose and lipid metabolism beyond its anti-inflammatory role. Therap. Adv. Endocrinol. Metab. 5(4):77–85. doi:10.1177/2042018814547204
Asmussen K, Andersen V, Bendixen G, Schiodt M, Oxholm P (1996) A new model for classification of disease manifestations in primary Sjogren’s syndrome: evaluation in a retrospective long-term study. J Intern Med 239(6):475–482
Ng WF, Bowman SJ (2010) Primary Sjogren’s syndrome: too dry and too tired. Rheumatology (Oxford) 49(5):844–853. doi:10.1093/rheumatology/keq009
Ambrosetti A, Zanotti R, Pattaro C, Lenzi L, Chilosi M, Caramaschi P, Arcaini L, Pasini F, Biasi D, Orlandi E, D’Adda M, Lucioni M, Pizzolo G (2004) Most cases of primary salivary mucosa-associated lymphoid tissue lymphoma are associated either with Sjoegren syndrome or hepatitis C virus infection. Br J Haematol 126(1):43–49. doi:10.1111/j.1365-2141.2004.04993.x
Both T, Hoorn EJ, Zietse R, van Laar JA, Dalm VA, Brkic Z, Versnel MA, van Hagen PM, van Daele PL (2015) Prevalence of distal renal tubular acidosis in primary Sjogren’s syndrome. Rheumatology (Oxford) 54(5):933–939. doi:10.1093/rheumatology/keu401
Brito-Zeron P, Akasbi M, Bosch X, Bove A, Perez-De-Lis M, Diaz-Lagares C, Retamozo S, Gandia M, Perez-Alvarez R, Soto-Cardenas MJ, Siso A, Valls-Sole J, Graus F, Ramos-Casals M (2013) Classification and characterisation of peripheral neuropathies in 102 patients with primary Sjogren’s syndrome. Clin Exp Rheumatol 31(1):103–110
Deheinzelin D, Capelozzi VL, Kairalla RA, Barbas Filho JV, Saldiva PH, de Carvalho CR (1996) Interstitial lung disease in primary Sjogren’s syndrome. Clinical-pathological evaluation and response to treatment. Am J Respir Crit Care Med 154(3 Pt 1):794–799. doi:10.1164/ajrccm.154.3.8810621
Jara LJ, Navarro C, Brito-Zeron Mdel P, Garcia-Carrasco M, Escarcega RO, Ramos-Casals M (2007) Thyroid disease in Sjogren’s syndrome. Clin Rheumatol 26(10):1601–1606. doi:10.1007/s10067-007-0638-6
Pease CT, Shattles W, Barrett NK, Maini RN (1993) The arthropathy of Sjogren’s syndrome. Br J Rheumatol 32(7):609–613
Laing CM, Unwin RJ (2006) Renal tubular acidosis. J Nephrol 19(Suppl 9):S46–52
Bushinsky DA, Chabala JM, Gavrilov KL, Levi-Setti R (1999) Effects of in vivo metabolic acidosis on midcortical bone ion composition. Am J Physiol 277(5 Pt 2):F813–819
Bushinsky DA, Krieger NS, Geisser DI, Grossman EB, Coe FL (1983) Effects of pH on bone calcium and proton fluxes in vitro. Am J Physiol 245(2):F204–209
Aerts J, Vigouroux C, Fournier P, Cariou D, Pasquier P (1994) Osteomalacia of renal origin disclosing Gougerot-Sjogren syndrome. Rev Med Intern 15(1):43–47
Cherif E, Ben Hassine L, Kaoueche Z, Khalfallah N (2013) Osteomalacia as inaugural manifestation of Sjogren syndrome. BMJ Case Rep 2013. doi:10.1136/bcr-2013-201052
Domrongkitchaiporn S, Pongsakul C, Stitchantrakul W, Sirikulchayanonta V, Ongphiphadhanakul B, Radinahamed P, Karnsombut P, Kunkitti N, Ruang-raksa C, Rajatanavin R (2001) Bone mineral density and histology in distal renal tubular acidosis. Kidney Int 59(3):1086–1093. doi:10.1046/j.1523-1755.2001.0590031086.x
Weger W, Kotanko P, Weger M, Deutschmann H, Skrabal F (2000) Prevalence and characterization of renal tubular acidosis in patients with osteopenia and osteoporosis and in non-porotic controls. Nephrol Dial Transpl. 15(7):975–980
Arampatzis S, Ropke-Rieben B, Lippuner K, Hess B (2012) Prevalence and densitometric characteristics of incomplete distal renal tubular acidosis in men with recurrent calcium nephrolithiasis. Urol Res 40(1):53–59. doi:10.1007/s00240-011-0397-3
Pongchaiyakul C, Domrongkitchaiporn S, Stitchantrakul W, Chailurkit LO, Rajatanavin R (2004) Incomplete renal tubular acidosis and bone mineral density: a population survey in an area of endemic renal tubular acidosis. Nephrol Dial Transpl. 19(12):3029–3033. doi:10.1093/ndt/gfh534
Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, Fox PC, Fox RI, Kassan SS, Pillemer SR, Talal N, Weisman MH (2002) Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis 61(6):554–558
Zillikens MC, Uitterlinden AG, van Leeuwen JP, Berends AL, Henneman P, van Dijk KW, Oostra BA, van Duijn CM, Pols HA, Rivadeneira F (2010) The role of body mass index, insulin, and adiponectin in the relation between fat distribution and bone mineral density. Calcif Tissue Int 86(2):116–125. doi:10.1007/s00223-009-9319-6
Schousboe JT, Shepherd JA, Bilezikian JP, Baim S (2013) Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J. Clin. Densitom. 16 (4):455–466. doi:10.1016/j.jocd.2013.08.004
Enneman AW, Swart KM, van Wijngaarden JP, van Dijk SC, Ham AC, Brouwer-Brolsma EM, van der Zwaluw NL, Dhonukshe-Rutten RA, van der Cammen TJ, de Groot LC, van Meurs J, Lips P, Uitterlinden AG, Zillikens MC, van Schoor NM, van der Velde N (2015) Effect of vitamin B12 and folic acid supplementation on bone mineral density and quantitative ultrasound parameters in older people with an elevated plasma homocysteine level: B-PROOF, a randomized controlled trial. Calcif Tissue Int 96(5):401–409. doi:10.1007/s00223-015-9968-6
Brkic Z, Maria NI, van Helden-Meeuwsen CG, van de Merwe JP, van Daele PL, Dalm VA, Wildenberg ME, Beumer W, Drexhage HA, Versnel MA (2013) Prevalence of interferon type I signature in CD14 monocytes of patients with Sjogren’s syndrome and association with disease activity and BAFF gene expression. Ann Rheum Dis 72(5):728–735. doi:10.1136/annrheumdis-2012-201381
Walsh SB, Shirley DG, Wrong OM, Unwin RJ (2007) Urinary acidification assessed by simultaneous furosemide and fludrocortisone treatment: an alternative to ammonium chloride. Kidney Int 71(12):1310–1316. doi:10.1038/sj.ki.5002220
Haugen AJ, Peen E, Hulten B, Johannessen AC, Brun JG, Halse AK, Haga HJ (2008) Estimation of the prevalence of primary Sjogren’s syndrome in two age-different community-based populations using two sets of classification criteria: the Hordaland Health Study. Scand J Rheumatol 37(1):30–34. doi:10.1080/03009740701678712
Fox RI, Dixon R, Guarrasi V, Krubel S (1996) Treatment of primary Sjogren’s syndrome with hydroxychloroquine: a retrospective, open-label study. Lupus 5(Suppl 1):S31–36
Rihl M, Ulbricht K, Schmidt RE, Witte T (2009) Treatment of sicca symptoms with hydroxychloroquine in patients with Sjogren’s syndrome. Rheumatology (Oxford) 48(7):796–799. doi:10.1093/rheumatology/kep104
Lakshminarayanan S, Walsh S, Mohanraj M, Rothfield N (2001) Factors associated with low bone mineral density in female patients with systemic lupus erythematosus. J Rheumatol 28(1):102–108
Mok CC, Mak A, Ma KM (2005) Bone mineral density in postmenopausal Chinese patients with systemic lupus erythematosus. Lupus 14(2):106–112
Xiu Y, Xu H, Zhao C, Li J, Morita Y, Yao Z, Xing L, Boyce BF (2014) Chloroquine reduces osteoclastogenesis in murine osteoporosis by preventing TRAF3 degradation. J Clin Investig 124(1):297–310. doi:10.1172/JCI66947
Acknowledgments
We thank all patients and nurses that participated in this study. T.B. was supported by the Dutch Kidney Foundation and the Dutch Sjögren patient society.
Contributorship
All authors meet the criteria for contributorship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Tim Both, M. Carola Zillikens, Ewout J. Hoorn, Robert Zietse, Jan A. M. van Laar, Virgil A. S. H. Dalm, Cornelia M. van Duijn, Marjan A. Versnel, Naomi I. Maria, P. Martin van Hagen, and Paul L. A. van Daele have declare that they do not have conflict of interest.
Ethical approval
The study was approved by the Medical Ethics Committee of the Erasmus Medical Center (MEC-2013-075). Informed consent was obtained from every participant.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Both, T., Zillikens, M.C., Hoorn, E.J. et al. Bone Mineral Density in Sjögren Syndrome Patients with and Without Distal Renal Tubular Acidosis. Calcif Tissue Int 98, 573–579 (2016). https://doi.org/10.1007/s00223-016-0112-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0112-z