Abstract
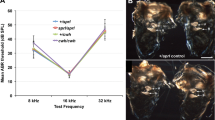
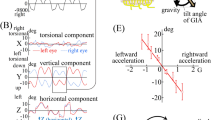
Genetically engineered mice are valuable models for elucidation of auditory and vestibular pathology. Our goal was to establish a comprehensive vestibular function testing system in mice using: (1) horizontal angular vestibulo-ocular reflex (hVOR) to evaluate semicircular canal function and (2) otolith-ocular reflex (OOR) to evaluate otolith organ function and to validate the system by characterizing mice with vestibular dysfunction. We used pseudo off-vertical axis rotation to induce an otolith-only stimulus using a custom-made centrifuge. For the OOR, horizontal slow-phase eye velocity and vertical eye position were evaluated as a function of acceleration. Using this system, we characterized hVOR and OOR in the caspase-3 (Casp3) mutant mice. Casp3 −/− mice had severely impaired hVOR gain, while Casp3 +/− mice had an intermediate response compared to WT mice. Evaluation of OOR revealed that at low-to-mid frequencies and stimulus intensity, Casp3 mutants and WT mice had similar responses. At higher frequencies and stimulus intensity, the Casp3 mutants displayed mildly reduced otolith organ-related responses. These findings suggest that the Casp3 gene is important for the proper function of the semicircular canals but less important for the otolith organ function.






Similar content being viewed by others
References
Al Deeb S, Al Moutaery K, Khan HA, Tariq M (2000) Exacerbation of iminodipropionitrile-induced behavioral toxicity, oxidative stress, and vestibular hair cell degeneration by gentamicin in rats. Neurotoxicol Teratol 22:213–220
Anagnostopoulos AV (2002) A compendium of mouse knockouts with inner ear defects. Trends Genet TIG 18:499
Anastasopoulos D, Haslwanter T, Fetter M, Dichgans J (1998) Smooth pursuit eye movements and otolith-ocular responses are differently impaired in cerebellar ataxia. Brain 121:1497–1505
Angelaki DE, Hess BJ (1996) Three-dimensional organization of otolith-ocular reflexes in rhesus monkeys. I. Linear acceleration responses during off-vertical axis rotation. J Neurophysiol 75:2405–2424
Benson AJ, Barnes GR (1973) Responses to rotating linear acceleration vectors considered in relation to a model of the otolith organs. In: Fifth symposium on the role of the vestibular organs in space exploration, NASA, Washington, DC.
Beraneck M, Cullen KE (2007) Activity of vestibular nuclei neurons during vestibular and optokinetic stimulation in the alert mouse. J Neurophysiol 98:1549–1565
Brettler SC, Rude SA, Quinn KJ, Killian JE, Schweitzer EC, Baker JF (2000) The effect of gravity on the horizontal and vertical vestibulo-ocular reflex in the rat. Exp Brain Res 132:434–444
Burt CW, Schappert SM (2004) Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1999–2000. Vital Health Stat 13(13):1–70
Cohen B, Suzuki JI, Raphan T (1983) Role of the otolith organs in generation of horizontal nystagmus: effects of selective labyrinthine lesions. Brain Res 276:159–164
Darlot C, Denise P (1988) Nystagmus induced by off-vertical rotation axis in the cat. Exp Brain Res 73:78–90
Elidan J, Sela M, Liebner E, Sohmer H (1991) Short latency vestibular evoked response to angular acceleration impulse in human beings. Otolaryngol Head Neck Surg 105:353–359
Faulstich BM, Onori KA, du Lac S (2004) Comparison of plasticity and development of mouse optokinetic and vestibulo-ocular reflexes suggests differential gain control mechanisms. Vis Res 44:3419–3427
Fekete DM (1999) Development of the vertebrate ear: insights from knockouts and mutants. Trends Neurosci 22:263–269
Fetter M (2007) Vestibulo-ocular reflex. Dev Ophthalmol 40:35–51
Furman JM, Redfern MS (2001) Effect of aging on the otolith-ocular reflex. J Vestib Res Equilib Orientat 11:91–103
Furman JM, Schor RH, Kamerer DB (1993) Off-vertical axis rotational responses in patients with unilateral peripheral vestibular lesions. Ann Otol Rhinol Laryngol 102:137–143
Guedry FE Jr (1965) Orientation of the rotation-axis relative to gravity: its influence on nystagmus and the sensation of rotation. Acta Oto-laryngol 60:30–48
Haslwanter T, Jaeger R, Mayr S, Fetter M (2000) Three-dimensional eye-movement responses to off-vertical axis rotations in humans. Exp Brain Res 134:96–106
Hess BJ, Dieringer N (1990) Spatial organization of the maculo-ocular reflex of the rat: responses during off-vertical axis rotation. Eur J Neurosci 2:909–919
Janeke JB, Jongkees LB, Oosterveld WJ (1970) Relationship between otoliths and nystagmus. Acta Oto-laryngol 69:1–6
Jones TA, Jones SM (1999) Short latency compound action potentials from mammalian gravity receptor organs. Hear Res 136:75–85
Jones SM, Subramanian G, Avniel W, Guo Y, Burkard RF, Jones TA (2002) Stimulus and recording variables and their effects on mammalian vestibular evoked potentials. J Neurosci Methods 118:23–31
Jones GE, Balaban CD, Jackson RL, Wood KA, Kopke RD (2003) Effect of trans-bullar gentamicin treatment on guinea pig angular and linear vestibulo-ocular reflexes. Exp Brain Res 152:293–306
Jones SM, Erway LC, Johnson KR, Yu H, Jones TA (2004) Gravity receptor function in mice with graded otoconial deficiencies. Hear Res 191:34–40
Jones SM, Johnson KR, Yu H, Erway LC, Alagramam KN, Pollak N, Jones TA (2005) A quantitative survey of gravity receptor function in mutant mouse strains. J Assoc Res Otolaryngol JARO 6:297–310
Jones SM, Jones TA, Johnson KR, Yu H, Erway LC, Zheng QY (2006) A comparison of vestibular and auditory phenotypes in inbred mouse strains. Brain Res 1091:40–46
Jones SM, Robertson NG, Given S, Giersch AB, Liberman MC, Morton CC (2011a) Hearing and vestibular deficits in the Coch(−/−) null mouse model: comparison to the Coch(G88E/G88E) mouse and to DFNA9 hearing and balance disorder. Hear Res 272:42–48
Jones TA, Jones SM, Vijayakumar S, Brugeaud A, Bothwell M, Chabbert C (2011b) The adequate stimulus for mammalian linear vestibular evoked potentials (VsEPs). Hear Res 280:133–140
Kaufman GD (2002) Video-oculography in the gerbil. Brain Res 958:472–487
Kerber KA, Meurer WJ, West BT, Fendrick AM (2008) Dizziness presentations in U.S. emergency departments, 1995–2004. Acad Emerg Med 15:744–750
Kimpo RR, Raymond JL (2007) Impaired motor learning in the vestibulo-ocular reflex in mice with multiple climbing fiber input to cerebellar Purkinje cells. J Neurosci 27:5672–5682
Lee SI, Conrad T, Jones SM, Lagziel A, Starost MF, Belyantseva IA, Friedman TB, Morell RJ (2013) A null mutation of mouse Kcna10 causes significant vestibular and mild hearing dysfunction. Hear Res 300:1–9
Makishima T, Hochman L, Armstrong P, Rosenberger E, Ridley R, Woo M, Perachio A, Wood SJ (2011) Inner ear dysfunction in caspase-3 deficient mice. BMC Neurosci 12:102
Maruta J, Simpson JI, Raphan T, Cohen B (2001) Orienting otolith-ocular reflexes in the rabbit during static and dynamic tilts and off-vertical axis rotation. Vis Res 41:3255–3270
Migliaccio AA, Meierhofer R, Della Santina CC (2011) Characterization of the 3D angular vestibulo-ocular reflex in C57BL6 mice. Exp Brain Res 210:489–501
Mira E (2008) Improving the quality of life in patients with vestibular disorders: the role of medical treatments and physical rehabilitation. Int J Clin Pract 62:109–114
Mock B, Jones TA, Jones SM (2011) Gravity receptor aging in the CBA/CaJ strain: a comparison to auditory aging. J Assoc Res Otolaryngol JARO 12:173–183
Morishita H, Makishima T, Kaneko C, Lee YS, Segil N, Takahashi K, Kuraoka A, Nakagawa T, Nabekura J, Nakayama K, Nakayama KI (2001) Deafness due to degeneration of cochlear neurons in caspase-3-deficient mice. Biochem Biophys Res Commun 284:142–149
Niven JI, Hixson WC, Correia MJ (1966) Elicitation of horizontal nystagmus by periodic linear acceleration. Acta Oto-laryngol 62:429–441
Oommen BS, Stahl JS (2008) Eye orientation during static tilts and its relationship to spontaneous head pitch in the laboratory mouse. Brain Res 1193:57–66
Plotnik M, Elidan J, Mager M, Sohmer H (1997) Short latency vestibular evoked potentials (VsEPs) to linear acceleration impulses in rats. Electroencephalogr Clin Neurophys 104:522–530
Rabbath G, Necchi D, de Waele C, Gasc JP, Josset P, Vidal PP (2001) Abnormal vestibular control of gaze and posture in a strain of a waltzing rat. Exp Brain Res 136:211–223
Robertson NG, Jones SM, Sivakumaran TA, Giersch AB, Jurado SA, Call LM, Miller CE, Maison SF, Liberman MC, Morton CC (2008) A targeted Coch missense mutation: a knock-in mouse model for DFNA9 late-onset hearing loss and vestibular dysfunction. Hum Mol Genet 17:3426–3434
Rodionov V, Elidan J, Sela M, Nitzan M, Sohmer H (1996) Vertical plane short and middle latency vestibular evoked potentials in humans. Ann Otol Rhinol Laryngol 105:43–48
Romand R, Krezel W, Beraneck M, Cammas L, Fraulob V, Messaddeq N, Kessler P, Hashino E, Dolle P (2013) Retinoic acid deficiency impairs the vestibular function. J Neurosci 33:5856–5866
Sheykholeslami K, Megerian CA, Zheng QY (2009) Vestibular evoked myogenic potentials in normal mice and Phex mice with spontaneous endolymphatic hydrops. Otol Neurotol 30:535–544
Stahl JS (2002) Calcium channelopathy mutants and their role in ocular motor research. Ann N Y Acad Sci 956:64–74
Stahl JS, Oommen BS (2008) Eye hyperdeviation in mouse cerebellar mutants is comparable to the gravity-dependent component of human downbeat nystagmus. Prog Brain Res 171:503–508
Stahl JS, Thumser ZC, Oommen BS (2012) The ataxic mouse as a model for studying downbeat nystagmus. J Vestib Res Equilib Orientat 22:221–241
Sugita-Kitajima A, Azuma M, Hattori K, Koizuka I (2007) Evaluation of the otolith function using sinusoidal off-vertical axis rotation in patients with benign paroxysmal positional vertigo. Neurosci Lett 422:81–86
Sung K, Reschke MF (1994) A four dimensional video eye movement tracking system. Aviat Space Environ Med 65:464
Takahashi K, Kamiya K, Urase K, Suga M, Takizawa T, Mori H, Yoshikawa Y, Ichimura K, Kuida K, Momoi T (2001) Caspase-3-deficiency induces hyperplasia of supporting cells and degeneration of sensory cells resulting in the hearing loss. Brain Res 894:359–367
Woo M, Hakem R, Soengas MS, Duncan GS, Shahinian A, Kagi D, Hakem A, McCurrach M, Khoo W, Kaufman SA, Senaldi G, Howard T, Lowe SW, Mak TW (1998) Essential contribution of caspase 3/CPP32 to apoptosis and its associated nuclear changes. Genes Dev 12:806–819
Wuyts FL, Furman J, Vanspauwen R, Van de Heyning P (2007) Vestibular function testing. Curr Opin Neurol 20:19–24
Yang TH, Young YH (2005) Click-evoked myogenic potentials recorded on alert guinea pigs. Hear Res 205:277–283
Yoshida H, Kong YY, Yoshida R, Elia AJ, Hakem A, Hakem R, Penninger JM, Mak TW (1998) Apaf1 is required for mitochondrial pathways of apoptosis and brain development. Cell 94:739–750
Zhao X, Jones SM, Yamoah EN, Lundberg YW (2008) Otoconin-90 deletion leads to imbalance but normal hearing: a comparison with other otoconia mutants. Neuroscience 153:289–299
Acknowledgments
We thank J. Stahl (Case Western Reserve University) for discussions and review of the manuscript, and B. Lavery and J. Anderson for technical assistance and discussions of the project. Casp3 mutant mice were a gift from Dr. Minna Woo (University of Toronto). Apaf1 mutant mice were a gift from Dr. Tak Mak (University of Toronto). PA was supported by the Jeane B. Kempner fellowship. Research reported in this publication was supported by the National Institute on Deafness and Other Communication Disorders of the National Institutes of Health under Award Number K08DC011540 to TM. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Armstrong, P.A., Wood, S.J., Shimizu, N. et al. Preserved otolith organ function in caspase-3-deficient mice with impaired horizontal semicircular canal function. Exp Brain Res 233, 1825–1835 (2015). https://doi.org/10.1007/s00221-015-4254-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-015-4254-4