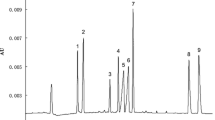
Abstract
International limits have been established for metal impurities in cosmetics to prevent overexposure to heavy metal ions. Sweeping via dynamic chelation was developed using capillary electrophoresis to analyze lead (Pb), cadmium (Cd) and mercury (Hg) impurities in cosmetics. The sweeping via dynamic chelation mechanism involves a large volume of metal ions being swept by a small quantity of chelating agents that were electrokinetically injected into the capillary to chelate metal ions and increase the detection sensitivity. The optimized conditions were as follows: Firstly, the capillary was rinsed by a 0.6 mM TTAB solution to reverse the EOF. The sample solution, which was diluted using 25 mM ammonium acetate (pH 6.0), was injected into the capillary using a pressure of 3.5 psi for 99.9 s. Then, EDTA was injected at −25 kV for 1 min from the EDTA buffer (25 mM ammonium acetate containing 0.6 mM TTAB and 5 mM EDTA), and the metal ions were swept and stacked simultaneously. Finally, the separation was performed at −20 kV using a separation buffer (100 mM ammonium acetate (pH 6.0)). A small quantity of chelating agents introduced into the capillary could yield 33-, 50- and 100-fold detection improvements for Pb, Cd and Hg, respectively, more sensitive than conventional capillary zone electrophoresis. Correlation coefficients greater than 0.998 indicated that this method exhibited good linearity. The relative standard deviation and relative error were less than 8.7%, indicating high precision and accuracy. The recovery value of the homemade lotion, which was employed to simulate the real sample matrix, was 93-104%, which indicated that the sample matrix does not affect the quantitative results. Finally, commercial cosmetics were employed to demonstrate the feasibility of the method to determine Pb, Cd and Hg without complicated sample pretreatment.

The procedure of analyzing metal ions in cosmetics by sweeping via dynamic chelation




Similar content being viewed by others
Abbreviations
- AA:
-
Atomic absorption
- AES:
-
Atomic emission spectroscopy
- Cd:
-
Cadmium
- CTAB:
-
Cetyltrimethyl ammonium bromide
- DTAB:
-
Dodecyl trimethylammonium bromide
- Hg:
-
Mercury
- HPLC:
-
High-performance liquid chromatography
- ICP-AES/MS:
-
Inductively coupled plasma atomic emission spectroscopy/mass spectrometry
- LIF:
-
Laser induced fluorescence
- LOD:
-
Limit of detection
- Pb:
-
Lead
- TTAB:
-
Tetradecyltrimethylammonium bromide
- UV:
-
Ultraviolet
References
Charter E, Dueckman M, Kurychak A, Martyn B, Nnebe N, Raymer B. Heavy metal hazard: the health risks pf hidden heavy metals on face makeup. Toronto, Canada: Environmental defence; 2011.
Chan TY. Inorganic mercury poisoning associated with skin-lightening cosmetic products. Clin Toxicol. 2011;49:886–91.
Borowska S, Brzoska MM. Metals in cosmetics: implications for human health. J Appl Toxicol. 2015;35:551–72.
Gidlow DA. Lead toxicity. Occup Med. 2015;65:348–56.
Bernhoft RA. Cadmium toxicity and treatment. Scientific World J. 2013, ID 394652
Bocca B, Pino A, Alimonti A, Forte G. Regul Toxicol Pharmacol. 2014;68:447–67.
Narin I, Soylak M, Elci L, Doğan M. Determination of trace metal ions by AAS in natural water samples after preconcentration of pyrocatechol violet complexes on an activated carbon column. Talanta. 2002;52:1041–6.
Ding H, Wang J, Dorsey JG, Caruso JA. Arsenic speciation by micellar liquid chromatography with inductively coupled plasma mass spectrometric detection. J Chromatogr A. 1995;694:425–31.
Rahmalan A, Zshari M, Marsin Sanagi M. M. Rashid. Determination of heavy metals in air particulate matter by ion chromatography. J Chromatogr A. 1996;739:233–9.
Corr JJ, Anacleto JF. Analysis of inorganic species by capillary electrophoresis-mass spectrometry and ion exchange chromatography-mass spectrometry using an ion spray source. Anal Chem. 1996;68:2155–63.
Huang YM, Whang CW. Capillary electrophoresis of arsenic compounds with indirect fluorescence detection. Electrophoresis. 1998;19:2140–4.
Regan FB, Meaney MP, Lunte SM. Determination of metal ions by capillary electrophoresis using on-column complexation with 4-(2-pyridylazo)resorcinol following trace enrichment by peak stacking. J Chromatogr B Biomed Appl. 1994;657:409–17.
Timerbaev AR, Semenova OP, Jandik P, Bonn GK. Metal ion capillary electrophoresis with direct UV detection effect of a charged surfactant on the migration behaviour of metal chelates. J Chromatogr A. 1994;671:419–27.
Motomizu S, Oshima M, Matsuda SY, Obata Y, Tanaka H. Separation and determination of alkaline-earth metal ions as UV absorbing chelates with EDTA by capillary electrophoresis. Determination of calcium and magnesium in water and serum samples. Anal Sci. 1992;8:619–25.
Haumann I, Bächann K. On-column chelation of metal ions in capillary zone Electrophoresis. J Chromatogr A. 1995;717:385–91.
Baraj B, Martínez M, Sastre A, M.Aguilar. Simultaneous determination of Cr (III), Fe (III), Cu (II) and Pb (II) as UV-absorbing EDTA complexes by capillary zone electrophoresis. J Chromatogr A. 1995; 695: 103–111.
Conradi S, Vogt C, Wittrisch H, Knobloch G, Werner G. Capillary electrophoretic separation of metal ions using complex forming equilibria of different stabilities. J Chromatogr A. 1996;745:103–9.
Liu W, Lee HK. Simultaneous analysis of lead, mercury and selenium species by capillary electrophoresis with combined ethylenediaminetetraacetic acid complexation and field-amplified stacking injection. Electrophoresis. 1999;20:2475–83.
Quirino JP, Haddad PR. Separation and sweeping of metal ions with EDTA in CZE-ESI-MS. J Sep Sci. 2011:34: 2872-78
Wang T, Li SFY. Migration behavior of alkali and alkaline-earth metal ion-EDTA complexes and quantitative analysis of magnesium in real samples by capillary electrophoresis with indirect ultraviolet detection. J Chromatogr A. 1995;707:343–53.
Breadmore MC, Macka M, Haddad PR. Theoretical migration model for micellar capillary electrophoresis and its application to the separation of anionic metal complexes of HEDTC and CDTA. Anal Chem. 1999;71:1826–33.
Isoo K, Terabe S. Metal complex separation with on-line sample preconcentration in micellar electrokinetic chromatography. Anal Sci. 2005;21:43–7.
Padarauskas A, Schwedt G. Capillary electrophoresis in metal analysis: Investigations of multi-elemental separation of metal chelates with aminopolycarboxylic acids. J Chromatogr A. 1997;773:351–60.
Shi Y, Fritz JS. Separation of metal ions by capillary electrophoresis with a complexing electrolyte. J Chromatogr A. 1993;640:473–9.
Lee YH, Lin TI. Determination of metal cations by capillary electrophoresis effect of background carrier and completing agents. J Chromatogr A. 1994;675:227–36.
Shakulashvili N, Faller T. H. Engelhardt. Simultaneous determination of alkali, alkaline earth and transition metal ions by capillary electrophoresis with indirect UV detection. J Chromatogr A. 2000;895:205–12.
Petr J, Gerstmann S, Frank H. Determination of some heavy metal cations in molten snow by transient isotachophoresis/capillary zone electrophoresis. J Sep Sci. 2006;29:2256–60.
Lopez CE, Castro JM, Gonzalez V, et al. Determination of metal ions in algal solution samples by capillary electrophoresis. J Chromatogr Sci. 1998;36:352–6.
Shi M, Gao Q, Feng J, Lu Y. Analysis of inorganic cations in honey by capillary zone electrophoresis with indirect UV detection. J Chromatogr Sci. 2012;50:547–52.
Piovezan M, Costa ACO, Jager AV, de Oliveira MAL, Micke GA. Development of a fast capillary electrophoresis method to determine inorganic cations in biodiesel samples. Anal Chim Acta. 2010;673:200–5.
Suarez-Luque S, Mato I, Huidobro JF, Simal-Lozano J. Rapid capillary zone electrophoresis method for the determination of metal cations in beverages. Talanta. 2006;68:1143–7.
Chiari M. Enhancement of selectivity in capillary electrophoretic separations of metals and ligands through complex formation. J Chromatogr A. 1998;805:1–15.
Quirino JP, Terabe S. Exceeding 5000-Fold Concentration of Dilute Analytes in Micellar Electrokinetic Chromatography. Science. 1998;282:465–8.
Isoo K, Terabe S. Analysis of metal ions by sweeping via dynamic complexation and cation-selective exhaustive injection in capillary electrophoresis. Anal Chem. 2003;75:6789–98.
Cocke DL, Schennach R, Yu Z. The surface properties of tetradecyltrimethylammonium bromide observed by capillary electrophoresis. J Chromatogr Sci. 2002;40:187–90.
Harakuwe AH, Haddad PR. Manipulation of separation selectivity in capillary zone electrophoresis of anionic solutes. Trends Analyt Chem. 2001;20:375–85.
Harakuwe AH, Haddad PR, Buchberger W. Optimisation of separation selectivity in capillary zone electrophoresis of inorganic anions using binary cationic surfactant mixtures. J Chromatogr A. 1994;685:161–5.
Acknowledgements
We gratefully acknowledge the support of the Ministry of Science and Technology of Taiwan (MOST 105-2113-M-037-014) and the partial support of the Kaohsiung Medical University “Aim for the Top Universities Grant” (No. KMU-TP104PR05) for funding this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study did not involve research on human participants or animals.
Conflict of interest
The authors have declared no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 402 kb)
Rights and permissions
About this article
Cite this article
Chen, KL., Jiang, SJ. & Chen, YL. Determining lead, cadmium and mercury in cosmetics using sweeping via dynamic chelation by capillary electrophoresis. Anal Bioanal Chem 409, 2461–2469 (2017). https://doi.org/10.1007/s00216-017-0193-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0193-1