Abstract
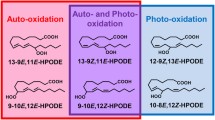
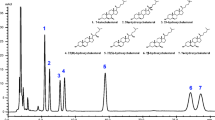
To elucidate the role of enzymatic lipid peroxidation in disease pathogenesis and in food deterioration, we recently achieved stereoselective analysis of phosphatidylcholine hydroperoxide (PCOOH) possessing 13S-hydroperoxy-9Z,11E-octadecadienoic acid (13(S)-9Z,11E-HPODE) using HPLC-MS/MS with a CHIRALPAK OP (+) column. Because enzymatic oxidation progresses concurrently with auto-oxidation, we need to distinguish them further. Here, we attempted such an analysis. First, we used lipoxygenase, linoleic acid, and lysophosphatidylcholine (LPC) to synthesize the enzymatic oxidation product 13(S)-9Z,11E-HPODE PC, and the auto-oxidation products 13(RS)-9Z,11E-HPODE PC and 13(RS)-9E,11E-HPODE PC, which were used as standards to test the ability of various columns to separate the enzymatic oxidation product from auto-oxidation products. Separation was achieved by connecting in series two columns with different properties: CHIRALPAK OP (+) and CHIRALPAK IB-3. The CHIRALPAK OP (+) column separated 13(R)-9Z,11E-HPODE PC and 13(S)-9Z,11E-HPODE PC, whereas CHIRALPAK IB-3 enabled separation of 13(S)-9Z,11E-HPODE PC and 13(RS)-9E,11E-HPODE PC. The results for the analysis of both enzymatically oxidized and auto-oxidized lecithin (an important phospholipid mixture in vivo and in food) indicate that our method would be useful for distinguishing enzymatic oxidation and auto-oxidation reactions. Such information will be invaluable for elucidating the involvement of PCOOH in disease pathogenesis and in food deterioration.






Similar content being viewed by others
Abbreviations
- 16:0 LPC:
-
1-palmitoyl-2-hydroxy-sn-glycero-3-phosphocholine
- 16:0/18:2 PC:
-
1-palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine
- C18:
-
octadecylsilane
- CD3OD:
-
methanol-d4
- CSP:
-
chiral stationary phase
- DCC:
-
dicyclohexylcarbodiimide
- DMAP:
-
4-dimethylaminopyridine
- HPETE:
-
hydroperoxyeicosatetraenoic acid
- HPODE:
-
hydroperoxyoctadecadienoic acid
- LA:
-
linoleic acid
- LOX:
-
lipoxygenase
- LPC:
-
lysophosphatidylcholine
- MxP:
-
2-methoxypropene
- NMR:
-
nuclear magnetic resonance
- PC:
-
phosphatidylcholine
- PCOOH:
-
phosphatidylcholine hydroperoxide
- PPTS:
-
pyridinium p-toluenesulfonate
- SRM:
-
selected reaction monitoring.
References
Berliner JA, Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20(5):707–27.
Kinoshita M, Oikawa S, Hayasaka K, Sekikawa A, Nagashima T, Toyota T, et al. Age-related increases in plasma phosphatidylcholine hydroperoxide concentrations in control subjects and patients with hyperlipidemia. Clin Chem. 2000;46:822–8.
Glass CK, Witztum JL. Atherosclerosis the road ahead. Cell. 2001;104(4):503–16.
Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stöckl J. Generation and biological activities of oxidized phospholipids. Antioxid Redox Signal. 2010;12(8):1009–59.
Ito J, Nakagawa K, Kato S, Miyazawa T, Kimura F, Miyazawa T. The combination of maternal and offspring high-fat diets causes marked oxidative stress and development of metabolic syndrome in mouse offspring. Life Sci. 2016;151:70–5.
Xu W, Takahashi Y, Sakashita T, Iwasaki T, Hattori H, Yoshimoto T. Low density lipoprotein receptor-related protein is required for macrophage-mediated oxidation of low density lipoprotein by 12/15-lipoxygenase. J Biol Chem. 2001;276(39):36454–9.
Funk CD, Cyrus T. 12/15-lipoxygenase, oxidative modification of LDL and atherogenesis. Trends Cardiovasc Med. 2001;11(3–4):116–24.
Kühn H, Borchert A. Regulation of enzymatic lipid peroxidation: the interplay of peroxidizing and peroxide reducing enzymes. Free Radic Biol Med. 2002;33(2):154–72.
Ito J, Nakagawa K, Kato S, Hirokawa T, Kuwahara S, Nagai T, et al. Direct separation of the diastereomers of phosphatidylcholine hydroperoxide bearing 13-hydroperoxy-9Z,11E-octadecadienoic acid using chiral stationary phase high-performance liquid chromatography. J Chromatogr A. 2015;1386:53–61.
Kato S, Nakagawa K, Suzuki Y, Asai A, Nagao M, Nagashima K, et al. Liquid chromatography-tandem mass spectrometry determination of human plasma 1-palmitoyl-2-hydroperoxyoctadecadienoyl-phosphatidylcholine isomers via promotion of sodium adduct formation. Anal Biochem. 2015;471:51–60.
Ibusuki D, Nakagawa K, Asai A, Oikawa S, Masuda Y, Suzuki T, et al. Preparation of pure lipid hydroperoxides. J Lipid Res. 2008;49(12):2668–77.
Kato S, Nakagawa K, Suzuki Y, Suzuki K, Mizuochi S, Miyazawa T. Preparation of 13 or 9-hydroperoxy-9Z,11E (9E,11E) or 10E,12Z (10E,12E)-octadecadienoic phosphatidylcholine hydroperoxide. J Oleo Sci. 2014;63(5):431–7.
Ito J, Mizuochi S, Nakagawa K, Kato S, Miyazawa T. Tandem mass spectrometry analysis of linoleic and arachidonic acid hydroperoxides via promotion of alkali metal adduct formation. Anal Chem. 2015;87(9):4980–7.
Schneider C, Yu Z, Boeglin WE, Zheng Y, Brash AR. Enantiomeric separation of hydroxy and hydroperoxy eicosanoids by chiral column chromatography. Methods Enzymol. 2007;433:145–57.
Garscha U, Nilsson T, Oliw EH. Enantiomeric separation and analysis of unsaturated hydroperoxy fatty acids by chiral column chromatography-mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872(1–2):90–8.
Younes AA, Mangelings D, Vander Heyden Y. Chiral separations in normal phase liquid chromatography: enantioselectivity of recently commercialized polysaccharide-based selectors. Part I: enantioselectivity under generic screening conditions. J Pharm Biomed Anal. 2011;55(3):414–23.
Geryk R, Kalíková K, Vozka J, Plecitá D, Schmid MG, Tesařová E. Enantioselective potential of chiral stationary phases based on immobilized polysaccharides in reversed phase mode. J Chromatogr A. 2014;1363:155–61.
Homann J, Lehmann C, Kahnt AS, Steinhilber D, Parnham MJ, Geisslinger G, et al. Chiral chromatography-tandem mass spectrometry applied to the determination of pro-resolving lipid mediators. J Chromatogr A. 2014;1360:150–63.
Song Y, Jing W, Yang F, Shi Z, Yao M, Yan R, et al. Simultaneously enantiospecific determination of (+)-trans-khellactone, (+/−)-praeruptorin A, (+/-)-praeruptorin B, (+)-praeruptorin E, and their metabolites, (+/−)-cis-khellactone, in rat plasma using online solid phase extraction-chiral LC-MS/MS. J Pharm Biomed Anal. 2014;88:269–77.
van Willem N, Mabel CT. Update on vegetable lecithin and phospholipid technologies. Lipid Sci Technol. 2008;110:472–86.
Cui L, Decker EA. Phospholipids in foods: prooxidants or antioxidants? J Sci Food Agric. 2016;96(1):18–31.
Acknowledgments
This work was supported by Grant-in-Aid for Scientific Research (B) Grant Number 15H04497.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest associated with this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 541 kb)
Rights and permissions
About this article
Cite this article
Ito, J., Nakagawa, K., Kato, S. et al. A novel chiral stationary phase HPLC-MS/MS method to discriminate between enzymatic oxidation and auto-oxidation of phosphatidylcholine. Anal Bioanal Chem 408, 7785–7793 (2016). https://doi.org/10.1007/s00216-016-9882-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-016-9882-4