Abstract
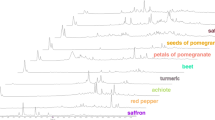
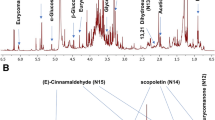
An untargeted metabolomic approach using liquid chromatography coupled to electrospray ionization time-of-flight mass spectrometry was developed in this work to identify novel markers for saffron authenticity which is an important matter related to consumer protection, quality assurance, active properties, and also economical impact (saffron is the most expensive spice). Metabolic fingerprinting of authentic and suspicious saffron samples from different geographical origin was obtained and analyzed. Different extracting protocols and chromatographic methodologies were evaluated to obtain the most adequate extracting and separation conditions. Using an ethanol/water mixture at pH 9.0 and an elution gradient with a fused core C18 column enabled obtaining the highest number of significant components between authentic and adulterated saffron. By using multivariate statistical analysis, predictive classification models for authenticity and geographical origin were obtained. Moreover, 84 and 29 significant metabolites were detected as candidates for markers of authenticity and geographical origin, respectively, from which only 34 metabolites were tentatively identified as authenticity markers of saffron, but none related to its geographical origin. Six characteristic compounds of saffron (kaempferol 3-O-glucoside, kaempferol 3-O-sophoroside, kaempferol 3,7-O-diglucoside, kaempferol 3,7,4′-O-triglucoside, kaempferol 3-O-sophoroside-7-O-glucoside, and geranyl-O-glucoside) were confirmed by comparing experimental MS/MS fragmentation patterns with those provided in scientific literature being proposed as novel markers of authenticity.

Metabolomic fingerprinting of saffron







Similar content being viewed by others
References
Melnyk JP, Wang S, Marcone MF (2010) Chemical and biological properties of the world’s most expensive spice: saffron. Food Res Int 43:1981–1989
Winterhalter P, Straubinger MS (2000) Saffron-renewed interest in an ancient spice. Food Rev Int 16:39–59
Hagh-Nazari S, Keifi N (2007) Saffron and various fraud manners in its production and trades. Acta Horticult 739:411–416
Kanti R, Sushma K, Ghandi R (2011) Adulteration and marketing of saffron (Crocus sativus L.). J Trop Med Plants 12:135–140
Carmona M, Zalacain A, Sanchez AM, Novella JL, Alonso GL (2006) Crocetin ester, picrocrocin and its related compounds present in Crocus sativus stigmas and Gardenia jasminoides fruits. Tentative identification of seven new compounds by LC-ESI-MS. J Agric Food Chem 54:973–979
Sanchez AM, Maggi L, Carmona M, Alonso GL (2011) In: Ebeler S (ed) Progress in authentication of food and wine. American Chemical Society, Washington DC
International standard ISO 3632-2: Saffron (Crocus sativus L.) test methods
International standard ISO 3632-1: Saffron (Crocus sativus L.) specification
Sabatino L, Scordino M, Gargano M, Belligno A, Traulo P, Gagliano G (2011) HPLC/PDA/ESI-MS evaluation of saffron (Crocus sativus L.) adulteration. Nat Prod Commun 6:1873–1876
Zalacain A, Ordoudi AS, Blazquez I, Diaz-olaza EM, Carmona M, Tsimldou MZ (2005) Screening method for the detection of artificial colours in saffron using derivative UV–vis spectrometry after precipitation of crocetin. Food Addit Contam 22:607–615
Sanchez AM, Carmona M, Zalacain A, Carot TM, Jabaloyes JM, Alonso GL (2008) Rapid determination of crocetin esters and picrocrocin from saffron spice (Crocus sativus L.) using UV visible spectrophotometry for quality control. J Agric Food Chem 56:3167–3175
Maggi L, Sanchez MA, Carmona M, Kanakis DC, Anastasaki E, Tarantilis AP (2011) Rapid determination of safranal in the quality control of saffron spice (Crocus sativus L). Food Chem 127:369–373
Zalacain A, Ordoudi SA, Blazquez I, Diaz-Plaza EM, Carmona M, Blazquez MZI (2005) Near-infrared spectroscopy in saffron quality control: determination of chemical composition and geographical origin. J Agric Food Chem 53:9337–9341
Assimiadis MK, Tarantilis PA, Polissiou MG (1998) UV–vis FT-Raman and 1H NMR spectroscopies of cisetrans carotenoids from saffron (Crocus sativus L). J Appl Spectrosc 52:519–522
Tarantilis PA, Polissiou MG (2004) Chemical analysis and antitumor activity of natural and semi-natural carotenoids of saffron. Acta Horticult 650:447–461
Zougagh M, Simonet BM, Rios A, Valcarcel M (2005) Use of nonaqueous capillary electrophoresis for the quality control of commercial saffron samples. J Chromatogr A 1085:293–298
Caballero-Ortega H, Pereda-Miranda R, Abdullaex FI (2007) HPLC quantification of mayor active components from 11 different saffron (Crocus sativus L.) sources. Food Chem 100:1126–1131
Lage M, Cantrell CL (2009) Quantification of saffron (Crocus sativus L.) metabolites crocins, picrocrocin and safranal for quality determination of the spice grown under different environmental Moroccan conditions. Sci Hortic 121:366–373
Loskutov AV, Beninger CW, Hoseld GL, Sink KC (2000) Development of an improved procedure for extraction and quantitation of safranal in stigmas of Crocus sativus L. using high performance liquid chromatography. Food Chem 69:87–95
Javanmardi N, Bagheri A, Moshtaghi N, Sharifi A, Hemati-Kakhki A (2011) Identification of safflower as a fraud in commercial saffron using RAPD/SCAR marker. J Cell Mol Res 3:31–37
Marieschi M, Torelli A, Bruni R (2012) Quality control of saffron (Crocus sativus L). Development of SCAR markers for the detection of plant adulterants used as bulking agents. J Agric Food Chem 60:10998–11004
Babaei S, Talebi M, Bahar M (2014) Developing an SCAR and ITS reliable multiplex PCR-based assay for safflower adulterant detection in saffron samples. Food Control 35:323–328
Torelli A, Marieschi M, Bruni R (2014) Authentication of saffron (Crocus sativus L.) in different processed, retail products by means of SCAR markers. Food Control 36:126–131
Cubero-Leon E, Peñalver R, Maquet A (2014) Review on metabolomics for food authentication. Food Res Int 60:95–104
Antignac JP, Courant F, Pinel G, Bichon E, Monteau F, Elliott C, Le Bizec B (2011) Mass spectrometry-based metabolomics applied to the chemical safety of food. Trends Anal Chem 30:292–301
Hu C, Xu G (2013) Mass-spectrometry-based metabolomics analysis for foodomics. Trends Anal Chem 52:36–46
Petrakis EA, Cagliani LR, Polissiou MG, Consonni R (2015) Evaluation of saffron (Crocus sativus L.) adulteration with plant adulterants by 1H NMR metabolite fingerprinting. Food Chem 173:890–896
Ciborowski M, Teul J, Martin-Ventura JL, Egido J (2012) Barbas C (2012) Metabolomics with LC–QTOF-MS permits the prediction of disease stage in aortic abdominal aneurysm based on plasma metabolic fingerprint. PLoS One 7(2), e31982
Westerhuis J, Hoefsloot H, Smit S, Vis D, Smilde A, van Velzen E, van Duijnhoven J, van Dorsten F (2004) Assessment of PLSDA cross validation. Metabolomics 4:81–89
El Rammouz R, Letisse F, Durand S, Portais JC, Moussa ZW, Fernandez X (2010) Analysis of skeletal muscle metabolome: evaluation of extraction methods for targeted metabolite quantification using liquid chromatography tandem mass spectrometry. Anal Biochem 398:169–177
Bruce S, Tavazzi I, Parisod V, Rezzi S, Kochhar S, Guy P (2009) Investigation of human blood plasma sample preparation for performing metabolomics using ultrahigh performance liquid chromatography/mass spectrometry. Anal Chem 81:3285–3296
Berrueta LA, Alfonso-Salces RM, Hérberger K (2007) Supervised pattern recognition in food analysis. J Chromatogr A 1158:196–214
Trygg J (2002) Orthogonal projections to latent structures (O-PLS). J Chemometr 16:119–128
Bylesjo M, Rantalainen M, Cloarec O, Nicholson JK, Holmes E, Trygg J (2006) OPLS discriminant analysis: combining the strengths of PLS‐DA and SIMCA classification. J Chemometr 20:341–351
Westerhuis JA, Velzen EJJ, Hoefsloot HCJ, Smilde AK (2010) Multivariate paired data analysis: multilevel PLSDA versus OPLDA. Metabolomics 6:119–128
van den Berg RA, Hoefsloot HC, Westerhuis JA, Smilde AK, van der Werf MJ (2006) Centering, scaling, and transformations: improving the biological information content of metabolomics data. BMC Genomics 7:142–147
Rubingh CM, Bijlsma S, Derks E, Bobeldijk I, Verheij ER (2006) Assessing the performance of statistical validation tools for megavariate metabolomics data. Metabolomics 2:53–61
Nørbæk R, Brandt K, Nielsen JK, Ørgaard M, Jacobsen N (2002) Flower pigment composition of Crocus species and cultivars used for a chemotaxonomic investigation. Biochem Syst Ecol 30:763–791
Goupy P, Vian MA, Chemat F, Caris-Veyrat C (2013) Identification and quantification of flavonols, anthocyanins and lutein diesters in tepals of Crocus sativus by ultra performance liquid chromatography coupled to diode array and ion trap mass spectrometry detections. Ind Crop Prod 44:496–510
Ferreres F, Llorach R, Gil-Izquierdo A (2004) Characterization of the interglycosidic linkage in di-, tri-, tetra- and pentaglycosylated flavonoids and differentiation of positional isomers by liquid chromatography/electrospray ionization tandem mass spectrometry. J Mass Spectrom 39:312–321
Serrano-Díaz J, Sańchez AM, Martínez-Tomé M, Winterhalter P, Alonso GL (2014) Flavonoid determination in the quality control of floral bioresidues from Crocus sativus L. J Agric Food Chem 62:3125–3133
Straubinger M, Jezussek M, Waibel R, Winterhalter P (1997) Novel glycosidic constituents from saffron. J Agric Food Chem 45:1678–1681
Montoro P, Tuberoso CIG, Maldini M, Cabras P, Pizza C (2008) Qualitative profile and quantitative determination of flavonoids from Crocus sativus L. petals by LC–MS/MS. Nat Prod Commun 3:2013–2016
Termentzi A, Kokkalou E (2008) LC–DAD–MS (ESI+) analysis and antioxidant capacity of Crocus sativus petal extracts. Planta Med 74:573–581
Vignolini P, Heimler D, Pinelli P, Ieri F, Sciullo A, Romani A (2008) Characterization of by-products of saffron (Crocus sativus L.) production. Nat Prod Commun 3:1959–1962
Carmona M, Sánchez AM, Ferreres F, Zalacain A, Tomás-Barberán F, Alonso GL (2007) Identification of the flavonoid fraction in saffron spice by LC/DAD/MS/MS: comparative study of samples from different geographical origins. Food Chem 100:445–450
Harborne JB (1999) In: Baxter H (ed) The handbook of natural flavonoids. Wiley, New York
Acknowledgments
The authors thank financial support from the Spanish Ministry of Science and Innovation (project CTQ2009-09022), the Comunidad of Madrid (Spain) and European funding from FEDER program (project S2013/ABI-3028, AVANSECAL-CM), and the University of Alcalá (project UAH2011/EXP-020). M.G.D. thanks the Ministry of Science and Innovation for his predoctoral contract (BES-2010-033465). The authors also thank the kind gift of saffron samples by Carmencita Company and the group of Prof. Coral Barbas for the one-month stay of M.G.D. in her laboratory to work with the different chemometric techniques used in this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guijarro-Díez, M., Nozal, L., Marina, M.L. et al. Metabolomic fingerprinting of saffron by LC/MS: novel authenticity markers. Anal Bioanal Chem 407, 7197–7213 (2015). https://doi.org/10.1007/s00216-015-8882-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8882-0