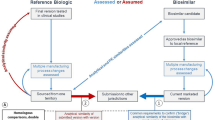
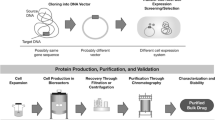
Abstract
Biosimilars are defined as biotherapeutic drugs that have been shown to have comparable quality, safety, and efficacy to the original product. Fuelled by the patent cliff in the next 5 years, the focus of the biopharmaceutical industry is gradually shifting towards production of biosimilars. Scientific and regulatory issues around development and approval of these biosimilars have been a topic of great interest and debate recently. Unlike the conventional small molecular weight drugs, biosimilars exhibit high complexity at the molecular level. Slight variations during the manufacturing of these complex protein molecules may lead to the significant changes in the safety and efficacy profile of the therapeutic product. Establishing comparability to the reference product is essential for successful approval of a biosimilar product. Analytical comparability provides the foundation to this exercise. This paper presents data from such an exercise involving use of several orthogonal analytical tools for establishing analytical comparability. Granulocyte colony-stimulating factor (GCSF/Filgrastim) expressed in Escherichia coli has been selected as the model protein. The approach would be of interest to those engaged in development and commercialization of biosimilars.

ᅟ







Similar content being viewed by others
References
Schellekens H (2004) Nat Biotechnol 22:1357–1359
Belsey MJ, Harris LM, Das RR, Chertkow J (2006) Nat Rev Drug Discov 5:535–536
Walsh G (2005) Trends Biotechnol 23:553–558
Rathore AS (2009) Trends Biotechnol 27:698–705
Weise M, Bielsky M-C, Smet KD, Ehmann F, Ekman N, Narayanan G, Heim H-K, Heinonen E, Ho K, Thorpe R, Vlemickx C, Wadhwa M, Schneider CK (2011) Nat Biotechnol 29:690–693
Berkowitz SA, Engen JR, Mazzeo JR, Jones GB (2012) Nat Rev Drug Discov 11:527–540
Chirino AJ, Mire-Sluis A (2004) Nat Biotechnol 22:1383–1389
Schneider CK, Kalinke U (2008) Nat Biotechnol 26:985–990
Kresse GB (2009) Eur J Pharm Biopharm 72:479–486
Skrlin A, Radic I, Vuletic M, Schwinke D, Runac D, Kusalic T, Paskvan I, Krsic M, Bratos M, Marinc S (2010) Biologicals 38:557–566
Ashton G (2001) Nat Biotechnol 19:307–311
Rathore AS, Low D (2010) BioPharm Int 23(11):34–40
Hagel L, Jagschies G, Sofer G (2007) Handbook of process chromatography, 2nd edn. Academic, New York
International Conference on Harmonisation Guideline Q8 (R1) (2008) Pharmaceutical Development. Geneva, Switzerland: International Conference on Harmonization of Technical Requirements for the Registration of Pharmaceuticals for Human Use.
US Food and Drug Administration. FDA guidance concerning demonstration of comparability of human biological products, including therapeutic biotechnology-derived products FDA, Rockville, MD, April 1996. http://www.fda.gov/cder/Guidance/compare.htm
US Food and Drug Administration. Revised guidance for industry: providing regulatory submissions to the Center for Biologics Evaluation and Research (CBER) in electronic format—biologics marketing applications [Biologics License Application (BLA), Product License Application (PLA)/Establishment License Application (ELA) and New Drug Application (NDA)] FDA, Rockville, MD, November 1999. http://www.cfsan.fda.gov/~dms/opaegui3.html
Petit I, Szyper-Karavitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, Sandbank J, Zipori D, Lapidot T (2002) Nat Immunol 3:687–694
Aritomi M, Kunishima N, Okamoto T, Kuroki R, Ota Y, Morikawa K (1999) Nature 401:713–717
Chu JW, Yin J, Wang DI, Trout BL (2004) Biochemistry 43:1019–1029
Chu JW, Brooks BR, Trout BL (2004) J Am Chem Soc 126:16601–16607
Lu HS, Fausset PR, Sotos LS, Clogston CL, Rohde MF, Stoney KS, Herman AC (1993) Protein Expr Purif 4:465–472
Guillon JM, Mechulam Y, Schmitter JM, Blanquet S, Fayat G (1992) J Bacteriol 174:4294
Schenauer MR, Flynn GC, Goetze AM (2012) Anal Biochem 428:150–157
Wang X, Junter AK, Mozier NM (2009) Biotechnol Bioeng 103:446–458
Valente KN, Schaefer AK, Kempton HR, Lenhoff AM, Lee KH (2014) Biotechnol J 9:87–99
Xie H, Chakraborty A, Ahn J, Yu YQ, Dakshinamoorthy DP, Chen W, Skilton J, Mazzeo J (2010) mAbs 2:379–394
Doneanu CE, Xenopoulos A, Fadgen K, Murphy J, Skilton SJ, Prentice H, Stapels M, Chen W (2012) mAbs 41:24–44
Gilar M, Xie H, Chakraborty A, Ahn J, Yu YQ, Chen W, Skilton J, Mazzeo J, Dakshinamoorthy DP (2010) Biopharm Int 2010:1–4
Bhambure R, Gupta D, Rathore AS (2013) J Chromatogr A 1314:188–198
Bhambure R, Sharma R, Gupta D, Rathore AS (2013) J Chromatogr A 1307:49–57
Bade P, Kotu SP, Rathore AS (2012) J Sep Sci 35:3160–3169
Filgrastim concentrated solution: European Pharmacopoeia Monograph 7:2015–2018
Cygnus application notes. http://cygnustechnologies.com/product_detail/bacterial/e-coli-hcp-elisa-kit.html
Invitrogen application notes. http://www.invitrogen.com/site/us/en/home/brands/Product-Brand/Quant-iT.html
Acknowledgments
Financial assistance from the Department of Biotechnology, Government of India, New Delhi, India is gratefully acknowledged. We are thankful to Waters Corporation, USA, for their help with analytical characterization of GCSF and to the National Institute of Biologicals, India, for performing the bioassay. We are also thankful to the High Impact Research Proposal Grant from IIT Delhi that contributed towards this project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Analysis of Biological Therapeutic Agents and Biosimilars with guest editor Karen Phinney.
Rights and permissions
About this article
Cite this article
Rathore, A.S., Bhambure, R. Establishing analytical comparability for “biosimilars”: filgrastim as a case study. Anal Bioanal Chem 406, 6569–6576 (2014). https://doi.org/10.1007/s00216-014-7887-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7887-4