Abstract
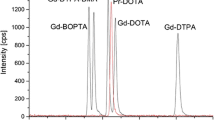
Hydrophilic interaction chromatography (HILIC) coupled with inductively coupled plasma mass spectrometry (ICP-MS) was optimized for speciation analysis of gadolinium-based contrast agents in environmental samples, in particular surface river waters and plants. Surface water samples from the Teltow channel, near Berlin, were investigated over a distance of 5 km downstream from the influx of a wastewater treatment plant. The total concentration of gadolinium increased significantly from 50 to 990 ng L−1 due to the influx of the contrast agents. After complete mixing with the river water, the concentration remained constant over a distance of at least 4 km. Two main substances [Dotarem® (Gd-DOTA) and Gadovist® (Gd-BT-DO3A)] have been identified in the river water using standards. A gadolinium-based contrast agent, possibly Gd-DOTA (Dotarem®), was also detected in water plant samples taken from the Teltow channel. Therefore, uptake of contrast agents [Gadovist® (Gd-BTDO3A), Magnevist® (Gd-DTPA), Omniscan® (Gd-DTPA-BMA), Dotarem® (Gd-DOTA), and Multihance® (Gd-BOPTA)] by plants was investigated in a model experiment using Lepidium sativum (cress plants). HILIC–ICP-MS was used for identification of different contrast agents, and a first approach for quantification using aqueous standard solutions was tested. For speciation analysis, all investigated contrast agents could be extracted from the plant tissues with a recovery of about 54 % for Multihance® (Gd-BOPTA) up to 106 % for Gadovist® (Gd-BT-DO3A). These experiments demonstrate that all contrast agents investigated are transported from the roots to the leaves where the highest content was measured.




Similar content being viewed by others
References
Bousquet JC, Saini S, Stark DD, Hahn PF, Nigam M, Wittenberg J, Ferrucci JT (1988) Gd-DOTA characterization of a new paramagnetic complex. Radiology 166(3):693–698
Weinmann HJ, Brasch RC, Press WR, Wesbey GE (1984) Characteristics of gadolinium-Dtpa complex—a potential NMR contrast agent. Am J Roentgenol 142(3):619–624
Ersoy H, Rybicki FJ (2007) Biochemical safety profiles of gadolinium-based extracellular contrast agents and nephrogenic systemic fibrosis. J Magn Reson Imaging 26(5):1190–1197. doi:10.1002/Jmri.21135
Idée J-M, Port M, Raynal I, Schaefer M, Le Greneur S, Corot C (2006) Clinical and biological consequences of transmetallation induced by contrast agents for magnetic resonance imaging: a review. Fundam Clin Pharmacol 20(6):563–576. doi:10.1111/j.1472-8206.2006.00447.x
Muroff LR (2001) Contrast: current agents and issues. Appl Radiol 30:5–7
Möller P, Morteani G, Dulski P (2003) Anomalous gadolinium, cerium, and yttrium contents in the Adige and Isarco River Waters and in the water of their tributaries (Provinces Trento and Bolzano/Bozen, NE Italy). Acta Hydrochimica Et Hydrobiologica 31(3):225–239. doi:10.1002/aheh.200300492
Bau M, Dulski P (1996) Anthropogenic origin of positive gadolinium anomalies in river waters. Earth Planet Sci Lett 143(1–4):245–255
Elbaz-Poulichet F, Seidel JL, Othoniel C (2002) Occurrence of an anthropogenic gadolinium anomaly in river and coastal waters of Southern France. Water Res 36(4):1102–1105
Morteani G, Moller P, Fuganti A, Paces T (2006) Input and fate of anthropogenic estrogens and gadolinium in surface water and sewage plants in the hydrological basin of Prague (Czech Republic). Environ Geochem Heal 28(3):257–264. doi:10.1007/s10653-006-9040-6
Knappe A, Möller P, Dulski P, Pekdeger A (2005) Positive gadolinium anomaly in surface water and ground water of the urban area Berlin, Germany. Chemie der Erde 65:167–189
Rabiet M, Togola A, Brissaud F, Seidel JL, Budzinski H, Elbaz-Poulichet F (2006) Consequences of treated water recycling as regards pharmaceuticals and drugs in surface and ground waters of a medium-sized Mediterranean catchment. Environ Sci Technol 40(17):5282–5288. doi:10.1021/Es060528p
Nozaki Y, Lerche D, Alibo DS, Tsutsumi M (2000) Dissolved indium and rare earth elements in three Japanese rivers and Tokyo Bay: evidence for anthropogenic Gd and In. Geochimica Et Cosmochimica Acta 64(23):3975–3982
Zhu YB, Hattori R, Rahmi D, Okuda S, Itoh A, Fujimori E, Umemura T, Haraguchi H (2005) Fractional distributions of trace metals in surface water of Lake Biwa as studied by ultrafiltration and ICP-MS. Bull Chem Soc Jpn 78(11):1970–1976. doi:10.1246/Bcsj.78.1970
Zhu Y, Hoshino M, Yamada H, Itoh A, Haraguchi H (2004) Gadolinium anomaly in the distributions of rare earth elements observed for coastal seawater and river waters around Nagoya City. Bull Chem Soc Jpn 77(10):1835–1842. doi:10.1246/Bcsj.77.1835
Verplanck PL, Taylor HE, Nordstrom DK, Barber LB (2005) Aqueous stability of gadolinium in surface waters receiving sewage treatment plant effluent, Boulder Creek, Colorado. Environ Sci Technol 39(18):6923–6929. doi:10.1021/es048456u
Kulaksiz S, Bau M (2007) Contrasting behaviour of anthropogenic gadolinium and natural rare earth elements in estuaries and the gadolinium input into the North Sea. Earth Planet Sci Lett 260(1–2):361–371. doi:10.1016/j.epsl.2007.06.016
Lawrence MG, Jupiter SD, Kamber BS (2006) Aquatic geochemistry of the rare earth elements and yttrium in the Pioneer River catchment, Australia. Mar Freshw Res 57(7):725–736. doi:10.1071/Mf05229
Nozaki Y, Lerche D, Alibo DS, Snidvongs A (2000) The estuarine geochemistry of rare earth elements and indium in the Chao Phraya River, Thailand. Geochimica Et Cosmochimica Acta 64(23):3983–3994
Hennebrüder K, Wennrich R, Mattusch J, Stärk H-J, Engewald W (2004) Determination of gadolinium in river water by SPE preconcentration and ICP-MS. Talanta 63(2):309–316
Kümmerer K, Helmers E (2000) Hospital effluents as a source of gadolinium in the aquatic environment. Environ Sci Technol 34(4):573–577. doi:10.1021/es990633h
Hemstrom P, Irgum K (2006) Hydrophilic interaction chromatography. J Sep Sci 29(12):1784–1821. doi:10.1002/jssc.200600199
Künnemeyer J, Terborg L, Nowak S, Scheffer A, Telgmann L, Tokmak F, Günsel A, Wiesmüller G, Reichelt S, Karst U (2008) Speciation analysis of gadolinium-based MRI contrast agents in blood plasma by hydrophilic interaction chromatography/electrospray mass spectrometry. Anal Chem 80(21):8163–8170. doi:10.1021/ac801264j
Künnemeyer J, Terborg L, Meermann B, Brauckmann C, Möller I, Scheffer A, Karst U (2009) Speciation analysis of gadolinium chelates in hospital effluents and wastewater treatment plant sewage by a novel HILIC/ICP-MS method. Environ Sci Technol 43(8):2884–2890
Raju CSK, Cossmer A, Scharf H, Panne U, Lück D (2010) Speciation of gadolinium based MRI contrast agents in environmental water samples using hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. J Anal At Spectrom 25(1):55–61
Kahakachchi CL, Moore DA (2010) Identification and characterization of gadolinium(III) complexes in biological tissue extracts. Metallomics 2(7):490–497
Yang LH, Wang XR, Sun H, Zhang HS (1999) The effect of EDTA on rare earth elements bioavailability in soil ecosystem. Chemosphere 38(12):2825–2833
Sun H, Wang XR, Wang Q, Wang HT, Wang LS, Chen YJ, Dai LM, Cao M (1997) The effects of chemical species on bioaccumulation of rare earth elements in wheat grown in nutrient solution. Chemosphere 35(8):1699–1707
Giussani A, Heinrichs U, Roth P, Werner E, Schramel P, Wendler A (1998) Biokinetic studies in humans with stable isotopes as tracers. Part 1: a methodology for incorporation of trace metals into vegetables. Isot Environ Heal Stud 34(3):291–296
Nowack B, Schwyzer I, Schulin R (2008) Uptake of Zn and Fe by wheat (Triticum aestivum var. Greina) and transfer to the grains in the presence of chelating agents (ethylenediaminedisuccinic acid and ethylenediaminetetraacetic acid). J Agric Food Chem 56(12):4643–4649. doi:10.1021/jf800041b
Nowack B, Schulin R, Robinson BH (2006) Critical assessment of chelant-enhanced metal phytoextraction. Environ Sci Technol 40(17):5225–5232. doi:10.1021/es0604919
Grcman H, Velikonja-Bolta S, Vodnik D, Kos B, Lestan D (2001) EDTA enhanced heavy metal phytoextraction: metal accumulation, leaching and toxicity. Plant Soil 235(1):105–114
Kretschy D, Koellensperger G, Hann S (2011) Stability assessment of different chelating moieties used for elemental labeling of bio-molecules. Metallomics 3:1304–1309. doi:10.1039/C1MT00114K
Möller P, Dulski P, Bau M, Knappe A, Pekdeger A, Sommer-von Jarmersted C (2000) Anthropogenic gadolinium as a conservative tracer in hydrology. J Geochem Explor 69:409–414
Bittner R (2010) Wasserwirtschaftlicher Monatsbericht Oktober 2010. Senatsverwaltung für Gesundheit, Umwelt und Verbraucherschutz, Berlin
Möller K, Burgschweiger J (2008) Wasserversorgungskonzept für Berlin und für das von der BWB versorgte Umland (Entwicklung bis 2040). Berliner Wasserbetriebe
Wild SR, Jones KC (1992) Organic chemicals entering agricultural soils in sewage sludges: screening for their potential to transfer to crop plants and livestock. Sci Total Environ 119:85–119
Yeo AR, Yeo ME, Flowers TJ (1987) The contribution of an apoplastic pathway to sodium uptake by rice roots in saline conditions. J Exp Bot 38(192):1141–1153
Isnard P, Lambert S (1988) Estimating bioconcentration factors from octanol-water partition coefficient and aqueous solubility. Chemosphere 17(1):21–34
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the topical collection Metallomics with guest editors Uwe Karst and Michael Sperling.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 170 KB)
Rights and permissions
About this article
Cite this article
Lindner, U., Lingott, J., Richter, S. et al. Speciation of gadolinium in surface water samples and plants by hydrophilic interaction chromatography hyphenated with inductively coupled plasma mass spectrometry. Anal Bioanal Chem 405, 1865–1873 (2013). https://doi.org/10.1007/s00216-012-6643-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-012-6643-x