Abstract
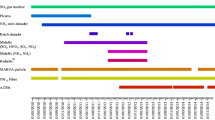
As part of the Baltimore PM2.5 Supersite study, intensive three-hourly continuous PM2.5 sampling was conducted for nearly 4 weeks in summer of 2002 and as well in winter of 2002/2003. Close to 120 individual organic compounds have been quantified separately in filter and polyurethane foam (PUF) plug pairs for 17 days for each sampling period. Here, the focus is on (1) describing briefly the new sampling system, (2) discussing filter/PUF plugs breakthrough experiments for semi-volatile compounds, (3) providing insight into phase distribution of semi-volatile organic species, and (4) discussing the impact of air pollution sampling time on human exposure with information on maximum 3- and 24-h averaged ambient concentrations of potentially adverse health effects causing organic pollutants. The newly developed sampling system consisted of five electronically controlled parallel sampling channels that are operated in a sequential mode. Semi-volatile breakthrough experiments were conducted in three separate experiments over 3, 4, and 5 h each using one filter and three PUF plugs. Valuable insight was obtained about the transfer of semi-volatile organic compounds through the sequence of PUF plugs and a cut-off could be defined for complete sampling of semi-volatile compounds on only one filter/PUF plug pair, i.e., the setup finally used during the seasonal PM2.5 sampling campaign. Accordingly, n-nonadecane (C19) with a vapor pressure (vp) of 3.25 × 10−4 Torr is collected with > 95% on the filter/PUF pair. Applied to phenanthrene, the most abundant the PAH sampled, phenanthrene (vp, 6.2 × 10−5 Torr) was collected completely in wintertime and correlates very well with three-hourly PM2.5 ambient concentrations. Valuable data on the fractional partitioning for semi-volatile organics as a function of season is provided here and can be used to differentiate the human uptake of an organic pollutant of interest via gas- and particle-phase exposure. Health effects studies often relay on PM2.5 exposure measurements taken over 24 h or longer. We found that maximum 3-h concentrations are frequently two to five times higher than that found for maximum 24-h concentrations, an important aspect when considering that short-term exposure to higher air pollution levels are more likely to overpower defense mechanisms in the human lung with subsequent adverse effects even at lower pollutant levels.







Similar content being viewed by others
Change history
17 August 2019
The authors would like to call the reader���s attention to the fact that unfortunately Orhan Sevimoglu���s affiliation was wrong in the original publication.
17 August 2019
The authors would like to call the reader���s attention to the fact that unfortunately Orhan Sevimoglu���s affiliation was wrong in the original publication.
References
Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C (2004) Inhal Toxicol 16:437–445
Oberdörster G, Maynard A, Donaldson K, Castranova K, Fitzpatrick J, Ausman K, Carter J, Karn B, Kreyling W, Lai D, Olin S, Monteiro-Riviere N, Warheit D, Yang H (2005) Particle and Fibre Toxicology 2005(2):8. doi:10.1186/1743-8977-2-8
Semmler-Behnke M, Takenaka S, Fersch S, Wenk A, Seitz J, Mayer O, Oberdorster G, Kreyling WG (2007) Environ Health Persp 115(5):728–733
Shimada A, Kawamura N, Okajima M, Kaewamatawong T, Inoue H, Morita T (2007) Toxicol Pathol 34:949–957
Pope CA, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD (2002) J Am Med Assoc 287:1132–1141
Peters A, Dockery DW, Muller JE, Mittleman MA (2001) Circulation 103:2810–2815
Nawrot TS, Perez L, Künzli N, Munters E, Nemery B (2011) Lancet 377:732–740
Vockens J (2003) Leith, D. Ann Occup Hyg 47:157–164
Suarez AE, Ondov JM (2002) Energy Fuel 16:562–568
Ondov JM, Buckley TJ, Hopke PK, Ogulei D, Parlange MB, Rogge WF, Squibb KS, Johnston MV, Wexler AS (2006) Atmos Environ 40:224–237
Oguleia D, Hopkea PK, Zhoua L, Paaterob P, Park SS, Ondov JM (2005) Atmos Environ 39:3751–3762
Park SS, Kleissl J, Harrison D, Kumar V, Nair NP, Adam M, Ondov J, Parlange M (2006) Aerosol Science Technol 40:845–860
Bidleman TF, Billings WN, Foreman WT (1986) Environ Sci Technol 20:1038–1043
McDow SR, Huntzicker JJ (1990) Atmos Environ 24:2563–2571
Wang L, Atkinson R, Arey J (2007) Atmos Environ 41:2025–2035
William BJ, Goldstein AH, Kreisberg NM, Hering SV (2010) PNAS 107:6676–6681
Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT (1991) Environ Sci Technol 25:1112–1125
Rogge WF, Hildemann LM, Mazurek MA, Cass GR, Simoneit BRT (1998) Environ Sci Technol 32:13–22
Liang F, Lu M, Birch ME, Keener TC, Liu Z (2006) J Chromatogr A 1114:145–153
Lippmann M (2009) J Exposure Sci Environ Epi 19:235–247
Acknowledgment
This work was supported by the US Environmental Protection Agency through cooperative agreement number R-82806301. Although the research described in this article has been funded wholly or in part by the US Environmental Protection Agency, it has not been subjected to the Agency’s required peer-and-policy review and therefore, does not necessarily reflect the views of the Agency and no official endorsement should be inferred.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in the special issue Aerosol Analysis with guest editor Ralf Zimmermann.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM. 1
(PDF 150 kb)
Rights and permissions
About this article
Cite this article
Rogge, W.F., Ondov, J.M., Bernardo-Bricker, A. et al. Baltimore PM2.5 Supersite: highly time-resolved organic compounds—sampling duration and phase distribution—implications for health effects studies. Anal Bioanal Chem 401, 3069–3082 (2011). https://doi.org/10.1007/s00216-011-5454-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5454-9