Abstract
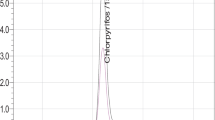
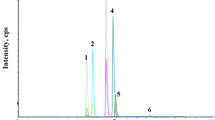
A new method for the analysis of three ecological insecticides, namely azadyrachtin, spinosad (sum of spinosyn A and spinosyn D) and rotenone, in produce and soil samples is presented. Investigated compounds are one of the most significant insecticides authorized for organic farming crop protection in many countries. Extraction of the pesticides from plant and soil matrices was performed by using a modified quick, easy, cheap, effective, rugged, and safe (QuEChERS) method. The method entailed a single extraction of the investigated compounds with acidified acetonitrile followed by a dispersive solid-phase extraction cleanup step prior to the final determination by reverse-phase ultra-performance liquid chromatography/tandem quadrupole mass spectrometry (UPLC-MS/MS). Validation studies were carried out on cabbage, tomato and soil samples. Recoveries of the spiked samples were in the range between 67% and 108%, depending on the matrix and the spiking level. Relative standard deviations for all matrix–compound combinations did not exceed 12%. The limits of quantification were ≤0.01 mg kg−1 in all cases, except for azadirachtin. The developed method was applied to the analysis of real samples originating from organic farming production.



Similar content being viewed by others
References
Scott RH, O'Brien K, Roberts L, Mordue W, Luntz M (1999) J Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 123:85–93
Sparks TC, Crouse GD, Durst G (2001) Pest Manag Sci 57:896–905
Salgado VL (1998) Pestic Biochem Physiol 60:91–102
Kowalska J ISOFAR/IFOAM/Organic World Congress Proceedings, Modena 18-20 June 2008, 1, 532-535
Kowalska J (2008) Ecol Chem Eng A 15:939–943
Tomline CDS (ed) (2000) The Pesticide Manual (12th ed). BCPC: Surrey, UK & the Royal Society of Chemistry: Cambridge, UK
West SD, Turner LG (2000) J Agric Food Chem 48:366–372
Jiménez JJ, Bernal JL, del Nozal MJ, Novo M, Higes M, Llorente J (2000) J Chromatogr A 871:67–73
Schwedler DA, Thomas AD, Yeh L-T (2000) J Agric Food Chem 48:5138–5145
Caboni P, Sarais G, Angioni A, Garau VL, Cabras P (2005) J Agric Food Chem 53:8644–8649
Sannino A (2007) Rapid Commun Mass Spectrom 21:2079–2086
Sarais G, Caboni P, Sarritzu E, Russo M, Cabras P (2008) J Agric Food Chem 56:2939–2943
Schaaf O, Jarvis AP, van der Esch SA, Giagnacovo G, Oldham NJ (2000) J Chromatogr A 886:89–97
Dai J, Yaylayan VA, Vijaya Raghavan GS, Pare JR (1999) J Agric Food Chem 47:3738–3742
Anastassiades M, Lehotay SJ, Stajnbaher D, Schenck FJ (2003) J AOAC Int 86:412–431
Walorczyk S (2007) J Chromatogr A 1165:200–212
Pizzutti IR, de Kok A, Zanella R, Adaime MB, Hiemstra M, Wickert C, Prestes OD (2007) J Chromatogr A 1142:123–136
European Commission, Method Validation and Quality Control Procedures for Pesticide Residues Analysis in Food and Feed, Document No. SANCO/2007/3131, Brussels, 31 October 2007
Commission Decision (2002/657/EC) of 12 August 2002 implementing Council Directive 96/23/EC concerning the performance of analytical methods and the interpretation of results, Off. J. Eur. Comm., No. L221/8,17 August 2002
Acknowledgments
This work was financially supported by the Polish Ministry of Science and Higher Education, Grant No. NN 310 4358 33r. The authors thank Stanisław Walorczyk, Ph.D., for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Drożdżyński, D., Kowalska, J. Rapid analysis of organic farming insecticides in soil and produce using ultra-performance liquid chromatography/tandem mass spectrometry. Anal Bioanal Chem 394, 2241–2247 (2009). https://doi.org/10.1007/s00216-009-2931-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-009-2931-5