Abstract
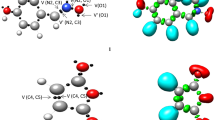
The mechanism of the non-catalysed and the MgBr2-catalysed [3+2] cycloaddition (32CA) reactions between C-methoxycarbonyl nitrone and 2-propen-1-ol has been theoretically investigated within the molecular electron density theory using DFT methods at the B3LYP/6-31G(d) computational level. Analysis of DFT reactivity indices allows explaining the role of the MgBr2 Lewis acid in the catalysed 32CA reaction. The 32CA reaction between C-methoxycarbonyl nitrone and 2-propen-1-ol takes place with a relative high activation enthalpy, 13.5 kcal mol−1, as a consequence of the non-polar character of this zw-type 32CA reaction. Coordination of the MgBr2 LA to C-methoxycarbonyl nitrone accelerates the corresponding zw-type 32CA reaction by taking place through a polar mechanism and with lower activation enthalpy, 8.5 kcal mol−1. Both 32CA reactions, which take place through a one-step mechanism, are completely meta regioselective and present low exo stereoselectivity, which increases in the catalysed process. Energy and non-covalent interaction analyses at the transition-state structures indicate that the formation of an intramolecular H–Br hydrogen bond in the catalysed process could be responsible for the exo selectivity experimentally observed.












Similar content being viewed by others
References
Huisgen R (1963) Angew Chem Int Ed 2:565–598
Padwa A (1984) 1, 3-Dipolar cycloaddition chemistry, vol 1-2. Wiley Interscience, New York
Padwa A (2002) Synthetic applications of 1,3-dipolar cycloaddition chemistry toward heterocycles and natural products, vol 59. Wiley, New York
Gothelf KV, Jorgensen KA (1998) Chem Rev 98:863–909
Tufariello JJ (1984) 1,3-Dipolar cycloaddition chemistry. A. Padwa, Wiley, New York
Torssell KBG (1988) Nitrile oxides, nitrones and nitronates in organic synthesis. VCH, New York
Tufariello JJ (1979) Acc Chem Res 12:396–403
Domingo LR, Sáez JA (2009) Org Biomol Chem 7:3576–3583
Domingo LR, Emamian SR (2014) Tetrahedron 70:1267–1273
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Tetrahedron 72:1524–1532
Domingo LR (2016) Molecules 21:1319
De Proft F, Geerlings P (2001) Chem Rev 101:1451–1464
Domingo LR, Ríos-Gutiérrez M, Pérez P (2016) Molecules 21:748
Becke AD, Edgecombe KE (1990) J Chem Phys 92:5397–5403
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-Garcia J, Cohen J, Yang AW (2010) J Am Chem Soc 132:6498–6506
Ríos-Gutiérrez M, Domingo LR, Pérez P (2015) RSC Adv 5:84797–84809
Domingo LR, Aurell MJ, Pérez P (2015) Tetrahedron 71:1050–1057
Domingo LR, Aurell MJ, Pérez P (2014) Tetrahedron 70:4519–4525
Kanemasa S (2010) Heterocycles 82:87–200
Simonsen KB, Bayón P, Hazell RG, Gothelf KV, Jorgensen KA (1999) J Am Chem Soc 121:3845–3853
Domingo LR (2000) Eur J Org Chem 2265–2272
Sousa CAD, Vale MLC, Garcia-Mera X, Rodriguez-Borges JE (2012) Tetrahedron 68:1682–1687
Nacereddinea AK, Layeb H, Chafaa F, Yahia W, Djerourou A, Domingo LR (2015) RSC Adv 5:64098–64105
Parr RG, von Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922–1924
Domingo LR, Chamorro E, Pérez P (2008) J Org Chem 73:4615–4624
Domingo LR, Pérez P (2011) Org Biomol Chem 9:7168–7175
S. Kanemasa S, Tsuruoka T (1995) Chem Lett 49–50
Geerlings P, De Proft F, Langenaeker W (2003) Chem Rev 103:1793–1874
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Hehre WJ, Radom L, Schleyer PVR, Pople J (1986) Ab initio Mol Orbital Theory. Wiley, New York
Schlegel HB (1982) J Comput Chem 2:214–218
Schlegel HB (1994) In modern electronic structure theory. In: Yarkony DR (ed) World Scientific Publishing, Singapore
Fukui K (1970) J Phys Chem 74:4161–4163
González C, Schlegel HB (1990) J Phys Chem 94:5523–5527
González C, Schlegel HB (1991) J Chem Phys 95:5853–5860
Tomasi J, Persico M (1994) Chem Rev 94:2027–2094
Simkin BY, Sheikhet I (1995) Quantum chemical and statistical theory of solutions—computational approach. Ellis Horwood, London
Cances E, Mennucci B, Tomasi J (1997) J Chem Phys 107:3032–3041
Cossi M, Barone V, Cammi R, Tomasi J (1996) Chem Phys Lett 255:327–335
Barone V, Cossi M, Tomasi J (1998) J Comput Chem 19:404–417
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735–746
Reed AE, Curtiss LA, Weinhold F (1988) Chem Rev 88:899–926
Lane JR, Contreras-Garcia J, Piquemal J-P, Miller BJ, Kjaergaard HG (2013) J Chem Theor Comput 9:3263–3266
Contreras-Garcia J, Johnson ER, Keinan S, Chaudret R, Piquemal J-P, Beratan DN, Yang W (2011) J Chem Theor Comput 7:625–632
Frisch MJ et al (2009) Gaussian 09, revision A.02. Gaussian Inc, Wallingford
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7514
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press, New York
Kohn W, Sham L (1965) J Phys Rev 140:1133–1138
Domingo LR, Pérez P, Sáez JA (2013) RSC Adv 3:1486–1494
Domingo LR, Chamorro E,Pérez P (2009) Eur J Org Chem 3036–3044
Domingo LR (2014) RSC Adv 4:32415–32428
Domingo LR, Aurell MJ, Pérez P, Contreras R (2002) Tetrahedron 58:4417–4423
Jaramillo P, Domingo LR, Chamorro E, Pérez P (2008) J Mol Struct (Theochem) 865:68–72
Benchouk W, Mekelleche SM, Silvi B, Aurell MJ, Domingo LR (2011) J Phys Org Chem 24:611–618
Ríos-Gutiérrez M, Pérez P, Domingo LR (2015) RSC Adv 5:58464–58477
Bader RFW, Essén HJ (1984) Chem Phys 80:1943–1960
Acknowledgements
This work has been supported by the Ministry of Economy and Competitiveness of the Spanish Government; Project CTQ2013-45646-P. M. Ríos-Gutiérrez also thanks the Ministry of Economy and Competitiveness for a pre-doctoral contract co-financed by the European Social Fund (BES-2014-068258). A.I. Adjieufack, I. M. Ndassa and J. K. Mbadcam are grateful to the Ministry of Higher Education of the Republic of Cameroon to finance the project with modernisation research allowance. The authors also thank the University of Yaoundé I and High Teacher Training College (Cameroon) for infrastructural facilities for generous allocation of computer time.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Adjieufack, A.I., Ndassa, I.M., Mbadcam, J.K. et al. Understanding the reaction mechanism of the Lewis acid (MgBr2)-catalysed [3+2] cycloaddition reaction between C-methoxycarbonyl nitrone and 2-propen-1-ol: a DFT study. Theor Chem Acc 136, 5 (2017). https://doi.org/10.1007/s00214-016-2028-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-2028-0