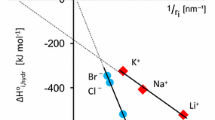
Abstract
The competition between \(\hbox {Na}^+\) and \(\hbox {K}^+\) with a protein for water was investigated by using Density Functional Theory (DFT) calculations. The optimized potential energy curves have been made in the DFT, together with balanced basis sets of split valence Def2-SV(P). Initially, calculations were done in order to know the organization of the hydration shell of the sodium and potassium ions, when up to sixteen molecules of water are added. The results indicate the structure and stability of these cations with water clusters. Then, this knowledge was used for the analysis of the hydrated protein when potassium or sodium cations approach them, showing that cation has a dehydration process more favorable energetically, and indicating for which cation, potassium or sodium is the competition with the protein for water more favorable.






Similar content being viewed by others
References
Pande A, Pande J, Asherie N, Lomakin A, Ogun O, King J, Benedek GB (2001) Crystal cataracts: Human genetic cataract caused by protein crystallization. Proc Natl Acad Sci USA 98:6116–6120
Dobson CM (2004) Principles of protein folding, misfolding and aggregation. Semin Cell Dev Biol 15:3–16 Protein Misfolding and Human Disease and Developmental Biology of the Retina
Ke H, van der Linde C, Lisy JM (2015) Insights into the structures of the gas-phase hydrated cations \({\text{ M }}^+ ({\text{ H }}_2 {\text{ O} })_n {\text{ Ar} }\) (M = Li, Na, K, Rb, and Cs; n = 3–5) using infrared photodissociation spectroscopy and thermodynamic analysis. J Phys Chem A 119:2037–2051
Yu H, Noskov SY, Roux B (2010) Two mechanisms of ion selectivity in protein binding sites. Proc Natl Acad Sci USA 107:20329–20334
Kim I, Allen TW (2011) On the selective ion binding hypothesis for potassium channels. Proc Natl Acad Sci USA 108:17963–17968
Britton KL, Baker PJ, Fisher M, Ruzheinikov S, Gilmour DJ, Bonete MJ, Ferrer J, Pire C, Esclapez J, Rice DW (2006) Analysis of protein solvent interactions in glucose dehydrogenase from the extreme halophile H aloferax mediterranei. (PDB ID: 2B5W). Proc Natl Acad Sci USA 103:4846–4851
Madern D, Ebel C (2007) Influence of an anion-binding site in the stabilization of halophilic malate dehydrogenase from H aloarcula marismortui. Biochimie 89:981–987
Ebel C, Faou P, Kernel B, Zaccai G (1999) Relative role of anions and cations in the stabilization of halophilic malate dehydrogenase. Biochemistry 38:9039–9047
Valle-Delgado J, Molina-Bolívar J, Galisteo-González F, Gálvez-Ruiz M (2011) Evidence of hydration forces between proteins. Curr Opin Colloid Interface Sci 16:572–578
Carrillo-Tripp M, Saint-Martin H, Ortega-Blake I (2003) A comparative study of the hydration of \(\text{ Na }^+\) and \(\text{ K }^+\) with refined polarizable model potentials. J Chem Phys 118:7062–7073
Ohtomo N, Arakawa K (1980) Neutron diffraction study of aqueous ionic solutions. ii. Aqueous solutions of sodium chloride and potassium chloride. Bull Chem Soc Jpn 53:1789–1794
Varma S, Rempe SB (2006) Coordination numbers of alkali metal ions in aqueous solutions. Biophys Chem 124:192–199 Ion Hydration Special Issue
Thomas M, Jayatilaka D, Corry B (2007) The predominant role of coordination number in potassium channel selectivity. Biophys J 93:2635–2643
Azam SS, Hofer TS, Randolf BR, Rode BM (2009) Hydration of sodium(i) and potassium(i) revisited: a comparative qm/mm and qmcf md simulation study of weakly hydrated ions. J Phys Chem A 113:1827–1834
Ramaniah LM, Bernasconi M, Parrinello M (1999) Ab initio molecular-dynamics simulation of \(\text{ K }^+\) solvation in water. J Chem Phys 111:1587–1591
Rao JS, Dinadayalane TC, Leszczynski J, Sastry GN (2008) Comprehensive study on the solvation of mono- and divalent metal cations: \(\text{Li}^+\), \(\text{Na}^+\), \(\text{K}^+\), \(\text{Be}^{2+}\), \(\text{Mg}^{2+}\) and \(\text{Ca}^{2+}\). J Phys Chem A 112:12944–12953
Neela Y, Mahadevi A, Sastry G (2013) First principles study and database analyses of structural preferences for sodium ion (\(\text{ Na }^+\)) solvation and coordination. Struct Chem 24:67–79
Chen H, Ruckenstein E (2015) Hydrated ions: from individual ions to ion pairs to ion clusters. J Phys Chem B 119:12671–12676
Mancinelli R, Botti A, Bruni F, Ricci MA, Soper AK (2007) Hydration of sodium, potassium, and chloride ions in solution and the concept of structure maker/breaker. J Phys Chem B 111:13570–13577
Rowley CN, Roux B (2012) The solvation structure of \(\text{ Na }^+\) and \(\text{ K }^+\) in liquid water determined from high level ab initio molecular dynamics simulations. J Chem Theor Comp 8:3526–3535
Becke AD (1993) Density-functional thermochemistry. iii. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange-correlation functional using the coulomb-attenuating method (cam-b3lyp). Chem Phys Lett 393:51–57
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297–3305
Hariharan PC, Pople JA (1973) The influence of polarization functions on molecular orbital hidrogenation energies. Theor Chim Acta 28:213
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. iii. The 3–21+g basis set for first-row elements, li- f. J Comput Chem 4:294–301
Case DA, Berryman JT, Betz RM, Cerutti DS, Cheatham III TE, Darden TA, Duke RE, Giese TJ, Gohlke H, Goetz AW, Homeyer N, Izadi S, Janowski P, Kaus J, Kovalenko A, Lee TS, LeGrand S, Li P, Luchko T, Luo R, Madej B, Merz KM, Monard G, Needham P, Nguyen H, Nguyen HT, Omelyan I, Onufriev A, Roe DR, Roitberg A, Salomon-Ferrer R, Simmerling CL, Smith W, Swails J, Walker RC, Wang J, Wolf RM, Wu X, York DM, Kollman PA (2015) Amber 2015. University of California, San Francisco
Hanwell M, Curtis D, Lonie D, Vandermeersch T, Zurek E, Hutchison G (2012) Avogadro: an advanced semantic chemical editor, visualization, and analysis platform. J Cheminform 4:17
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian-09 revision D.01. Gaussian Inc, Wallingford CT
Illingworth CJR, Furini S, Domene C (2010) Computational studies on polarization effects and selectivity in \(\text{ K }^+\) channels. J Chem Theor Comp 6:3780–3792
Acknowledgments
This work was partially supported by Spanish Ministry of Science and Technology Grant FIS2012-35880.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ferrer, J., San-Fabián, E. Competition for water between protein (from Haloferax mediterranei) and cations \(\hbox {Na}^+\) and \(\hbox {K}^+\): a quantum approach to problem. Theor Chem Acc 135, 228 (2016). https://doi.org/10.1007/s00214-016-1983-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-016-1983-9