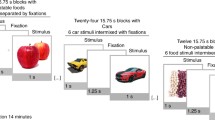
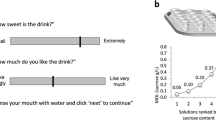
Abstract
Rationale
Endogenous opioids inhibit nociceptive processing and promote the experience of pleasure. It has been proposed that pain and pleasure lie at opposite ends of an affective spectrum, but the relationship between pain and pleasure and the role of opioids in mediating this relationship has not been tested.
Objectives
Here, we used obese individuals as a model of a dysfunctional opioid system to assess the role of the endogenous opioid peptide, beta-endorphin, on pain and pleasure sensitivity.
Methods
Obese (10M/10F) and age- and gender-matched non-obese (10M/10F) controls were included in the study. Pain sensitivity using threshold, tolerance, and subjective rating assessments and perceived sweet pleasantness using sucrose solutions were assessed in two testing sessions with placebo or the opioid antagonist, naltrexone (0.7 mg/kg body weight). Beta-endorphin levels were assessed in both sessions.
Results and conclusions
Despite having higher levels of baseline beta-endorphin and altered beta-endorphin-reactivity to naltrexone, obese individuals reported a similar increase in pain and decrease in pleasantness following naltrexone compared to non-obese individuals. Beta-endorphin levels did not correlate with pain or pleasantness in either group, but naltrexone-induced changes in pain and pleasantness were mildly correlated. Moreover, naltrexone-induced changes in pain were related to depression scores, while naltrexone-induced changes in sweet pleasantness were related to anxiety scores, indicating that pain and pleasantness are related, but influenced by different processes.




Similar content being viewed by others
References
Akil H, Watson SJ, Young E, et al. (1984) Endogenous opioids: biology and function. Annu Rev Neurosci 7:223–255. doi:10.1146/annurev.ne.07.030184.001255
al’Absi M, Wittmers L, Ellestad D, et al. (2004) Sex differences in pain and hypothalamic-pituitary-adrenocortical responses to opioid blockade. Psychosom Med 66:198–206. doi:10.1097/01.psy.000016250.81254.5d
Alvarez A, Singh PM, Sinha AC (2014) Postoperative analgesia in morbid obesity. Obes Surg 24:652–659. doi:10.1007/s11695-014-1185-2
Andreatta M, Fendt M, Mühlberger A, et al. (2012) Onset and offset of aversive events establish distinct memories requiring fear and reward networks. Learning & memory (Cold Spring Harbor, NY) 19:518–526. doi:10.1101/lm.026864.112
Arbisi PA, Billington CJ, Levine AS (1999) The effect of naltrexone on taste detection and recognition threshold
Badiani A, Rajabi H, Nencini P, Stewart J (2001) Modulation of food intake by the kappa opioid U-50,488H: evidence for an effect on satiation. Behav Brain Res 118:179–186
Becker HC (2012) Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research: Current Reviews 448–458
Berridge KC, Kringelbach ML (2013) Neuroscience of affect: brain mechanisms of pleasure and displeasure. Curr Opin Neurobiol 23:294–303. doi:10.1016/j.conb.2013.01.017
Berridge KC, Robinson TE, Aldridge JW (2009) Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 9:65–73. doi:10.1016/j.coph.2008.12.014
Bruehl S, Burns JW, Chung OY, Chont M (2012) What do plasma beta-endorphin levels reveal about endogenous opioid analgesic function? Eur J Pain 16:370–380. doi:10.1002/j.1532-2149.2011.00021.x
Bruijnzeel AW (2009) kappa-Opioid receptor signaling and brain reward function. Brain Res Rev 62:127–146. doi:10.1016/j.brainresrev.2009.09.008
Bucher HU, Moser T, Von Siebenthal K, Keel M, Wolf M, Duc G (1995) Sucrose reduces pain reaction to heel lancing in preterm infants: a placebo-controlled, randomized and masked study. Pediatr Res 38(3):332–335
Chieng B, Christie MJ (1994) Inhibition by opioids acting on μ‐receptors of GABAergic and glutamatergic postsynaptic potentials in single rat periaqueductal gray neurones in vitro. Br J Pharmacol 113(1):303–309
Davis JM, Lowy MT, Yim GKW, et al. (1983) Relationship between plasma concentrations of immunoreactive beta-endorphin and food intake in rats. Peptides 4:79–83
DiFeliceantonio AG, Mabrouk OS, Kennedy RT, Berridge KC (2012) Enkephalin surges in dorsal neostriatum as a signal to eat. Curr Biol 22:1918–1924. doi:10.1016/j.cub.2012.08.014
Drewnowski A, Krahn D, Demitrack M, et al. (1992) Taste responses and preferences for sweet high-fat foods—evidence for opioid involvement. Physiol Behav 51:371–379
Drolet G, Dumont EC, Gosselin I, et al. (2001) Role of endogenous opioid system in the regulation of the stress response. Prog Neuro-Psychopharmacol Biol Psychiatry 25:729–741
Fantino M, Hosotte J, Apfelbaum M (1986) An opioid antagonist, naltrexone, reduces preference for sucrose in humans. Am J Physiol 251:R91–R96
Fillingim RB, Kaplan L, Staud R, et al (2005) The A118G single nucleotide polymorphism of the μ-opioid receptor gene (OPRM1) is associated with pressure pain sensitivity in humans
Filliol D, Ghozland S, Chluba J, et al. (2000) Mice deficient for δ- and μ-opioid receptors exhibit opposing alterations of emotional responses. Nat Genet 25:195–200
Frid M, Singer G, Rana C (1979) Interactions between personal expectations and naloxone—effects on tolerance to ischemic pain. Psychopharmacology 65:225–231
Gonzalez JP, Brogden RN (1988) Naltrexone. A review of its pharmacodynamic and pharmacokinetic properties and therapeutic efficacy in the management of opioid dependence. Drugs 35:192–213
Gosnell BA, Levine AS (2009) Reward systems and food intake: role of opioids. Int J Obes 33(Suppl 2):S54–S58. doi:10.1038/ijo.2009.73
Hargreaves KM, Dionne RA, Mueller GP, et al. (1986) Naloxone, fentanyl, and diazepam modify plasma beta-endorphin levels during surgery. Clin Pharmacol Ther 40:165–171
Heinricher MM, Morgan MM, Fields HL (1992) Direct and indirect actions of morphine on medullary neurons that modulate nociception. Neuroscience 48(3):533–543
Hirakawa N, Tershner SA, Fields HL, Manning BH (2000) Bi-directional changes in affective state elicited by manipulation of medullary pain-modulatory circuitry. Neuroscience 100:861–871
Hollister LE, Johnson K, Boukhabza D, Gillespie HK (1981) Aversive effects of naltrexone in subjects not dependent on opiates. Drug Alcohol Depend 8:37–41
Hosobuchi Y, Adams JE, Linchitz R (1977) Pain relief by electrical stimulation of the central gray matter in humans and its reversal by naloxone. Science 197:183–186
Jackson A, Cooper SJ (1985) Effects of kappa opiate agonists on palatable food consumption in non-deprived rats, with and without food preloads. Brain Res Bull 15:391–396
Johansen JP, Fields HL, Manning BH (2001) The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A 98:8077–8082. doi:10.1073/pnas.141218998
Kalin NH, Shelton SE, Barksdale CM (1988) Opiate modulation of separation-induced distress in non-human primates. Brain Res 440:285–292. doi:10.1016/0006-8993(88)90997-3
Kampov-Polevoy AB, Ziedonis D, Steinberg ML, et al. (2003) Association between sweet preference and paternal history of alcoholism in psychiatric and substance abuse patients. Alcohol Clin Exp Res 27:1929–1936. doi:10.1097/01.ALC.0000099265.60216.23
Karayiannakis A, Syrigos K, Zbar A, et al. (1998) The effect of vertical banded gastroplasty on glucose-induced beta-endorphin response. J Surg Res 80:123–128. doi:10.1006/jsre.1998.5466 S0022-4804(98)95466-X [pii]
Khosla T, Lowe C (1967) Indices of obesity derived from body weight and height. British Journal of Preventive and Social Medicine 21:122–128
Koch M, Schmid A, Schnitzler HU (1996) Pleasure-attenuation of startle is disrupted by lesions of the nucleus accumbens. Neuroreport 7:1442–1446
Kringelbach ML, Berridge KC (2009) Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci (Regul Ed) 13:479–487. doi:10.1016/j.tics.2009.08.006
Kringelbach ML, O’Doherty J, Rolls ET, Andrews C (2003) Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 13:1064–1071
Lee YY, Kim KH, Yom YH (2007) Predictive models for post-operative nausea and vomiting in patients using patient-controlled analgesia. J Int Med Res 35(4):497–507
Liu N-J, Xu T, Xu C, et al. (1995) Cholecystokinin octapeptide reverses u-opioid-receptor-mediated inhibition of calcium current in rat dorsal root ganglion neurons. J Pharmacol Exp Ther 275:1293–1299
Loggia ML, Mogil JS, Bushnell MC (2008) Experimentally induced mood changes preferentially affect pain unpleasantness. J Pain 9:784–791. doi:10.1016/j.jpain.2008.03.014
Martin del Campo AF, Dowson JH, Herbert J, Paykel ES (1994) Effects of naloxone on diurnal rhythms in mood and endocrine function: a dose-response study in man. Psychopharmacology 114:583–590
Martinez-Guisasola J, Guerrero M, Alonso F, et al. (2001) Plasma beta-endorphin levels in obese and non-obese patients with polycystic ovary disease. Gynecol Endocrinol 15:14–22
McKendall M, Haier R (1983) Pain sensitivity and obesity. Psychiatry Res 8:119–125. doi:10.1016/0165-1781(83)90099-9 [pii]
McNair DM, Lorr M, Droppleman LF (1971) Profile of mood states
Mendelson JH, Ellingboe J, Keuhnle JC, Mello NK (1979) Effects of naltrexone on mood and neuroendocrine function in normal adult males. Psychoneuroendocrinology 3:231–236
Morley JE, Levine AS (1985) Dynorphin, an endogenous stimulator of feeding. Prog Clin Biol Res 192:293–300
Mucha RF, Herz A (1985) Motivational properties of kappa and mu opioid receptor agonists studied with place and taste preference conditioning. Psychopharmacology 86:274–280
Navratilova E, Xie JY, Meske D, et al. (2015) Endogenous opioid activity in the anterior cingulate cortex is required for relief of pain. J Neurosci 35:7264–7271. doi:10.1523/JNEUROSCI.3862-14.2015
Nicolas P, Hammonds R, Li C (1984) Beta-endorphin-induced analgesia is inhibited by synthetic analogs of beta-endorphin. Proc Natl Acad Sci U S A 81:3074–3077
O’Brien CP, Greenstein RA, Mintz J, Woody GE (1975) Clinical experience with naltrexone. Amer J Drug & Alcohol Abuse 2:365–377
Ookuma K, Barton C, York DA, Bray GA (1997) Effect of enterostatin and kappa-opioids on macronutrient selection and consumption. Peptides 18:785–791
Oyama T, Jin T, Yamaya R, et al. (1980) Profound analgesic effects of β-endorphin in man. Lancet 315:122–124. doi:10.1016/S0140-6736(80)90606-6
Pan ZZ, Tershner SA, Fields HL (1997) Cellular mechanism for anti-analgesic action of agonists of the kappa-opioid receptor. Nature 389:382–385. doi:10.1038/38730
Pfeiffer A, Branti V, Herz A, Emrich HM (1985) Psychotomimesis mediated by k opiate receptors. Science 233:774–776
Price DD, McGrath PA, Rafii A, Buckingham B (1983) The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain, 17(1):45–56
Price RC, Asenjo JF, Christou NV, et al. (2013) The role of excess subcutaneous fat in pain and sensory sensitivity in obesity. Eur J Pain 17:1316–1326. doi:10.1002/j.1532-2149.2013.00315.x
Price D, Gruen Von d A, Miller J, et al. (1985) A psychopyhsical analysis of morphine analgesia. Pain 22:261–269
Rainville P, Duncan GH, Price DD, et al. (1997) Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 277:968–971
Ramarao P, Bhargava HN (1989) Effects of kappa-opioid receptor agonists and morphine on food intake and urinary output in food-deprived and nondeprived rats. Pharmacol Biochem Behav 33:375–380
Recant L, Voyles NR, Luciano M, Pert CB (1980) Naltrexone reduces weight gain, alters “B-endorphin,” and reduces insulin output from Ppancreatic eslets of genetically obese mice. Peptides 1:309–313
Segato FN, Castro-Souza C, Segato EN, Morato S, Coimbra NC (1997) Sucrose ingestion causes opioid analgesia. Braz J Med Biol Res 30(8):981–984
Scavo D, Barletta C, Buzzetti R, Vagiri D (1988) Effects of caloric restriction and exercise on B-endorphin, ACTH and cortisol circulating levels in obesity. Physiol Behav 42:65–68. doi:10.1016/0031-9384(88)90261-2 [pii]
Straneva PA, Maixner W, Light KC, et al. (2002) Menstrual cycle, beta-endorphins, and pain sensitivity in premenstrual dysphoric disorder. Health Psychol 21:358–367
Szyfelbein SK, Osgood PF, Carr DB (1985) The assessment of pain and plasma beta-endorphin immunoactivity in burned children. Pain 22:173–182
Takemori AE, Portoghese PS (1984) Comparative antagonism by naltrexone and naloxone of m, k, and d agonists. Eur J Pharmacol 104:101–104
Veith JL, Anderson J, Slade SA, et al. (1984) Plasma beta-endorphin, pain thresholds and anxiety levels across the human menstrual cycle. Physiol Behav 32:31–34
Villemure C, Slotnick B, Bushnell M (2003) Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain 106:101–108
Wood PB, Schweinhardt P, Jaeger E, et al. (2007) Fibromyalgia patients show an abnormal dopamine response to pain. Eur J Neurosci 25:3576–3582. doi:10.1111/j.1460-9568.2007.05623.x
Woolley JD, Lee BS, Kim B, Fields HL (2007) Opposing effects of intra-nucleus accumbens mu and kappa opioid agonists on sensory specific satiety. Neuroscience 146:1445–1452. doi:10.1016/j.neuroscience.2007.03.012
Yeomans M, Gray R (1996) Selective effects of naltrexone on food pleasantness and intake. Physiol Behav 60:439–446
Zahorska-Markiewicz B, Kucio C, Pyszkowska J (1983) Obesity and pain. Hum Nutr Clin Nutr 37:307–310
Zahorska-Markiewicz B, Zych P, Kucio C (1988) Pain sensitivity in obesity. Acta Psychiatr Scand 39:183–187
Acknowledgments
We thank Valerie Cotton, Willem McIssac, and Cecelia Webber for help with data acquisition and Samantha AG Backman and Tianzheng Lin for their help with the saliva assays.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the McGill University Ethics board in accordance with the Declaration of Helsinki (2013). Written informed consent was obtained before the first session.
Funding
No funding to report.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Supplementary Table 1
Naltrexone effects on current mood. Table S1 displays information on the Profile of Mood States (POMS) scale subscales for ‘elated-depressed’ and ‘composedanxious’, which were used as measures of current depressive mood and anxiety, respectively. Data are shown for obese and non-obese groups and for pre- and post-capsule administration measurements in placebo and naltrexone sessions. There were no significant main effects or interactions for POMS subscales. (DOCX 11 kb)
Supplementary Table 2
Naltrexone effects on pain and sweet pleasantness. The univariate posthoc tests from the significant multivariate analysis of variance (MANOVA) for the effect of naltrexone on the 20 pain measures are shown. Additionally, the result of the ANOVA for the effect of naltrexone on sweet pleasantness ratings is also shown. Means and standard deviations (SD) are shown for obese and non-obese groups in each condition as well as the pooled (obese and non-obese) data. Due to the lack of a difference between obese and non-obese groups, all univariate tests, as well as the ANOVA for sweet pleasantness, were performed on the pooled data. The majority of tests showed increased pain sensitivity in the naltrexone condition compared to placebo condition as indicated in the last column by an upward arrow (↑). Tests in which pain sensitivity or sweet pleasantness was decreased in the naltrexone compared to placebo condition are indicated by a downward arrow (↓). (DOCX 307 kb)
Supplementary Table 3
Reported side effects in placebo and naltrexone sessions. Table 3 displays the median and range of reported side effects at each time point for obese, non-obese, and the pooled results for both obese and non-obese. The ‘TOTAL’ score is the sum of all other side effects and has a maximum value of 28. All individual side effects were rated using a 5-point Likert scale: 0 = None, 1 = Weak, 2 = Moderate, 3 = Strong, 4 = Extremely strong; therefore, the maximum value for all individual side effects was 4. Total side effects significantly differed 3.5 hours after naltrexone administration compared to placebo (p < 0.05). ‘Tiredness’ was the only individual side effect that differed at this time point (p < 0.05). (DOCX 16 kb)
Rights and permissions
About this article
Cite this article
Price, R.C., Christou, N.V., Backman, S.B. et al. Opioid-receptor antagonism increases pain and decreases pleasure in obese and non-obese individuals. Psychopharmacology 233, 3869–3879 (2016). https://doi.org/10.1007/s00213-016-4417-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4417-4