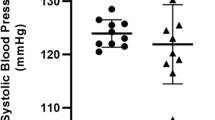
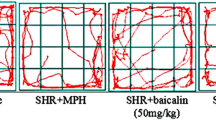
Abstract
Rationale
Dysregulation of noradrenergic and dopaminergic systems is involved in the pathology of attention deficit hyperactivity disorder (ADHD). Carbonic anhydrase (CA) has been reported to affect monoamine transmission in the central nervous system.
Objectives
The aim of this study is to investigate the effect of CA inhibitors on the hyperactivity and impulsivity of the spontaneously hypertensive rat (SHR), which is currently the best-validated animal model of ADHD.
Methods
SHRs and Wistar Kyoto rats at 6 to 8 weeks of age were pretreated with intraperitoneal injections of acetazolamide and methazolamide, both carbonic anhydrase inhibitors, before the behavior tests. The open-field locomotion test and the electro-foot shock aversive water drinking test were then applied to quantify their hyperactivity and impulsivity, respectively. The Morris water maze test, on the other hand, monitored their spatial learning.
Results
Acetazolamide and methazolamide significantly inhibited the hyperactivity of SHRs but had no effects in Wistar Kyoto rats. Acetazolamide also inhibited the impulsivity of SHRs. Low doses of acetazolamide had the greater inhibitory effects on the hyperactivity and impulsivity, but did not impair the spatial learning of SHRs.
Conclusions
This is the first study to show that carbonic anhydrase inhibitors can strain-specifically antagonize the hyperactivity and impulsivity of SHRs. Under a low dose of acetazolamide, there was no cognition impairment in SHRs. Carbonic anhydrase inhibitors may be the novel drugs for treatment for patients with ADHD.





Similar content being viewed by others
References
American Psychiatric Association (2013) Diagnostic and statistical manual of mental disorders: DSM-5. American Psychiatric Association, Arlington
Bari A, Robbins TW (2013) Inhibition and impulsivity: behavioral and neural basis of response control. Prog Neurobiol 108:44–79
Bergstom WH, Carzoli RF, Lombroso C, Davidson DT, Wallace WM (1952) Observations on the metabolic and clinical effects of carbonic-anhydrase inhibitors in epileptics. AMA Am J Dis Child 84:771–772
Bermejo PE, Ruiz-Huete C, Anciones B (2010) Zonisamide in managing impulse control disorders in Parkinson’s disease. J Neurol 257:1682–1685
Carmack SA, Howell KK, Rasaei K, Reas ET, Anagnostaras SG (2014) Animal model of methylphenidate’s long-term memory-enhancing effects. Learn Mem 21:82–89
Chaudieu I, St-Pierre JA, Quirion R, Boksa P (1994) GABAA receptor-mediated inhibition of N-methyl-d-aspartate-evoked [3H] dopamine release from mesencephalic cell cultures. Eur J Pharmacol 264:361–369
Cortese S, Holtmann M, Banaschewski T, Buitelaar J, Coghill D, Danckaerts M, Dittmann RW, Graham J, Taylor E, Sergeant J (2013) Practitioner review: current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. J Child Psychol Psychiatry 54:227–246
Dalley JW, Roiser JP (2012) Dopamine, serotonin and impulsivity. Neuroscience 215:42–58
Dalley JW, Everitt BJ, Robbins TW (2011) Impulsivity, compulsivity, and top-down cognitive control. Neuron 69:680–694
Del Campo N, Chamberlain SR, Sahakian BJ, Robbins TW (2011) The roles of dopamine and noradrenaline in the pathophysiology and treatment of attention-deficit/hyperactivity disorder. Biol Psychiatry 69:e145–e157
Dichter GS, Damiano CA, Allen JA (2012) Reward circuitry dysfunction in psychiatric and neurodevelopmental disorders and genetic syndromes: animal models and clinical findings. J Neurodev Disord 4:19
Gray WD, Rauh CE (1974) The anticonvulsant action of the carbonic anhydrase inhibitor methazolamide: possible involvement of a noradrenergic mechanism. Eur J Pharmacol 28:42–54
Jones Z, Dafny N (2013) Dose response effect of methylphenidate on ventral tegmental area neurons and animal behavior. Brain Res Bull 96:86–92
Katayama F, Miura H, Takanashi S (2002) Long-term effectiveness and side effects of acetazolamide as an adjunct to other anticonvulsants in the treatment of refractory epilepsies. Brain Dev 24:150–154
Kawata Y, Okada M, Murakami T, Mizuno K, Wada K, Kondo T, Kaneko S (1999) Effects of zonisamide on K+ and Ca2+ evoked release of monoamine as well as K+ evoked intracellular Ca2+ mobilization in rat hippocampus. Epilepsy Res 35:173–182
Kim P, Choi I, Pena IC, Kim HJ, Kwon KJ, Park JH, Han SH, Ryu JH, Cheong JH, Shin CY (2012) A simple behavioral paradigm to measure impulsive behavior in an animal model of attention deficit hyperactivity disorder (ADHD) of the spontaneously hypertensive rats. Biomol Ther (Seoul) 20:125–131
Laviolette SR, Gallegos RA, Henriksen SJ, van der Kooy D (2004) Opiate state controls bi-directional reward signaling via GABAA receptors in the ventral tegmental area. Nat Neurosci 7:160–169
Loring DW, Williamson DJ, Meador KJ, Wiegand F, Hulihan J (2011) Topiramate dose effects on cognition: a randomized double-blind study. Neurology 76:131–137
Meneses A, Perez-Garcia G, Ponce-Lopez T, Tellez R, Gallegos-Cari A, Castillo C (2011) Spontaneously hypertensive rat (SHR) as an animal model for ADHD: a short overview. Rev Neurosci 22:365–371
Murata M (2010) Zonisamide: a new drug for Parkinson’s disease. Drugs Today 46:251–258
National Research Council (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington, DC
Okada M, Kaneko S, Hirano T, Mizuno K, Kondo T, Otani K, Fukushima Y (1995) Effects of zonisamide on dopaminergic system. Epilepsy Res 22:193–205
Paterson NE, Ricciardi J, Wetzler C, Hanania T (2011) Sub-optimal performance in the 5-choice serial reaction time task in rats was sensitive to methylphenidate, atomoxetine and d-amphetamine, but unaffected by the COMT inhibitor tolcapone. Neurosci Res 69:41–50
Robbins T, Curran H, de Wit H (2012) Special issue on impulsivity and compulsivity. Psychopharmacology (Berl) 219:251–252
Russell VA (2011) Overview of animal models of attention deficit hyperactivity disorder (ADHD). Curr Protoc Neurosci Chapter 9: Unit9 35
Sagvolden T (2000) Behavioral validation of the spontaneously hypertensive rat (SHR) as an animal model of attention-deficit/hyperactivity disorder (AD/HD). Neurosci Biobehav Rev 24:31–39
Sagvolden T, Johansen E (2012) Rat models of ADHD. In: Stanford C, Tannock R (eds) Behavioral neuroscience of attention deficit hyperactivity disorder and its treatment (current topics in behavioral neurosciences). Springer Press, Berlin, pp 301–315
Sagvolden T, Xu T (2008) l-Amphetamine improves poor sustained attention while d-amphetamine reduces overactivity and impulsiveness as well as improves sustained attention in an animal model of attention-deficit/hyperactivity disorder (ADHD). Behav Brain Funct 4:3
Scassellati C, Bonvicini C, Faraone SV, Gennarelli M (2012) Biomarkers and attention-deficit/hyperactivity disorder: a systematic review and meta-analyses. J Am Acad Child Adolesc Psychiatr 51(1003–1019), e20
Shaw M, Hodgkins P, Caci H, Young S, Kahle J, Woods AG, Arnold LE (2012) A systematic review and analysis of long-term outcomes in attention deficit hyperactivity disorder: effects of treatment and non-treatment. BMC Med 10:99
Sonuga-Barke EJ (2005) Causal models of attention-deficit/hyperactivity disorder: from common simple deficits to multiple developmental pathways. Biol Psychiatry 57:1231–1238
Spencer T, Biederman J, Wilens T, Harding M, O’Donnell D, Griffin S (1996) Pharmacotherapy of attention-deficit hyperactivity disorder across the life cycle. J Am Acad Child Adolesc Psychiatry 35:409–432
Sun MK, Alkon DL (2001) Pharmacological enhancement of synaptic efficacy, spatial learning, and memory through carbonic anhydrase activation in rats. J Pharmacol Exp Ther 297:961–967
Ting-A-Kee R, Vargas-Perez H, Mabey JK, Shin SI, Steffensen SC, van der Kooy D (2013) Ventral tegmental area GABA neurons and opiate motivation. Psychopharmacology (Berl) 227:697–709
Tominaga M, Nagatomo I, Uchida M, Hashiguchi W, Akasaki Y, Takigawa M (2001) Alterations of nitric oxide and monoamines in the brain of the EL mouse treated with phenobarbital and zonisamide. Psychiatry Clin Neurosci 55:311–318
Torchiana ML, Lotti VJ, Stone CA (1973) The anticonvulsant effect of carbonic anhydrase inhibitors in mice—a noradrenergic mechanism of action. Eur J Pharmacol 21:343–349
Umehara M, Ago Y, Fujita K, Hiramatsu N, Takuma K, Matsuda T (2013a) Effects of serotonin-norepinephrine reuptake inhibitors on locomotion and prefrontal monoamine release in spontaneously hypertensive rats. Eur J Pharmacol 702:250–257
Umehara M, Ago Y, Kawanai T, Fujita K, Hiramatsu N, Takuma K, Matsuda T (2013b) Methylphenidate and venlafaxine attenuate locomotion in spontaneously hypertensive rats, an animal model of attention-deficit/hyperactivity disorder, through alpha2-adrenoceptor activation. Behav Pharmacol 24:328–331
Verrotti A, Loiacono G, Di Sabatino F, Zaccara G (2013) The adverse event profile of zonisamide: a meta-analysis. Acta Neurol Scand 128:297–304
Westover AN, Halm EA (2012) Do prescription stimulants increase the risk of adverse cardiovascular events?: a systematic review. BMC Cardiovasc Disord 12:41
Willcutt EG (2012) The prevalence of DSM-IV attention-deficit/hyperactivity disorder: a meta-analytic review. Neurotherapeutics 9:490–499
Xiao C, Zhou C, Li K, Ye J-H (2007) Presynaptic GABAA receptors facilitate GABAergic transmission to dopaminergic neurons in the ventral tegmental area of young rats. J Physiol 580:731–743
Yang MT, Chien WL, Lu DH, Liou HC, Fu WM (2013) Acetazolamide impairs fear memory consolidation in rodents. Neuropharmacology 67:412–418
Acknowledgments
The authors would like to thank Sau-Pin Won, MD, a native English speaker, for English editing of this manuscript to the standards of the journal. This work was supported by the Ministry of Science and Technology, Taiwan (NSC 102-2321-B-002-065).
Disclosure/conflicts of interest
The authors have no conflict of interest to be declared.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
Photograph of electro-foot shock aversive water drinking test (EFSDT) apparatus. The black Plexiglas box is divided into three compartments (start area, water area, and free area). During the testing phase (day 3), an electro-foot shock (2 mA, 0.5 s from the stimulator) was given whenever the rats licked water from the bottle for at least 5 s. Details of the apparatus and experimental protocol are described in the “Methods” section. (GIF 66 kb)
Rights and permissions
About this article
Cite this article
Yang, MT., Lu, DH., Chen, JC. et al. Inhibition of hyperactivity and impulsivity by carbonic anhydrase inhibitors in spontaneously hypertensive rats, an animal model of ADHD. Psychopharmacology 232, 3763–3772 (2015). https://doi.org/10.1007/s00213-015-4036-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-015-4036-5