Abstract
Rationale
Rats bred for high (HiS) and low (LoS) saccharin intake exhibit divergent behavioral responses to multiple drugs of abuse, with HiS rats displaying greater vulnerability to drug taking. Previous research indicates that this effect may be due to increased sensitivity to reward in HiS rats and to the aversive effects of acute drug administration in LoS rats.
Objective
The current study investigated whether HiS and LoS rats also exhibit different behavioral signs of withdrawal following one or repeated opiate exposures.
Methods
Emotional signs of opiate withdrawal were assessed with potentiation of the acoustic startle reflex and conditioned place aversion (CPA) in male and female HiS and LoS rats. Startle was measured before and 4 h after a 10-mg/kg injection of morphine on days 1, 2, and 7 of opiate exposure. CPA was induced with a 2-day, naloxone-precipitated conditioning paradigm. Somatic signs of withdrawal and weight loss were also measured.
Results
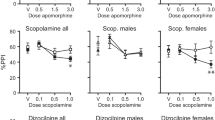
Male and female LoS rats exhibited lower startle potentiation than HiS rats on the seventh day of morphine exposure. LoS male rats also failed to develop a CPA to morphine withdrawal. No differences in physical withdrawal signs were observed between HiS and LoS rats, but males of both lines had more physical signs of withdrawal than females.
Conclusions
These results suggest that LoS rats are less vulnerable to the negative emotional effects of morphine withdrawal than HiS rats. A less severe withdrawal syndrome may contribute to decreased levels of drug taking in the LoS line.



Similar content being viewed by others
References
Anker JJ, Carroll ME (2010) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96
Aston-Jones G, Delfs JM, Druhan J, Zhu Y (1999) The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci 877:486–498
Avena NM, Rada P, Hoebel BG (2008) Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci Biobehav Rev 32:20–39
Azar MR, Jones BC, Schulteis G (2003) Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacol (Berl) 70:42–50
Badia Elder N, Kiefer SW, Dess NK (1996) Taste reactivity in rats selectively bred for high vs. low saccharin consumption. Physiol Behav 59:749–755
Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111:33–51
Bechara A, Nader K, van der Kooy D (1995) Neurobiology of withdrawal motivation: evidence for two separate aversive effects produced in morphine-naive versus morphine-dependent rats by both naloxone and spontaneous withdrawal. Behav Neurosci 109:91–105
Becker JB, Hu M (2008) Sex differences in drug abuse. Front Neuroendocrin 29:36–47
Beebe-Center JG, Black P, Hoffman AC, Wade M (1948) Relative per diem consumption as a measure of preference in the rat. J Comp Physiol Psych 41:239–251
Bozarth MA, Wise RA (1984) Anatomically distinct opiate receptor fields mediate reward and physical dependence. Science 224:516–517
Budney AJ, Vandrey RG, Hughes JR, Thostenson JD, Bursac Z (2008) Comparison of cannabis and tobacco withdrawal: severity and contribution to relapse. J Subst Abuse Treat 35:362–368
Cabral A, Ruggiero RN, Nobre MJ, Brandao ML, Castilho VM (2009) GABA and opioid mechanisms of the central amygdala underlie the withdrawal-potentiated startle from acute morphine. Prog Neuro-Psychoph 33:334–344
Carroll ME, Anker JJ (2010) Sex differences and ovarian hormones in animal models of drug dependence. Horm Behav 58:44–56
Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacol (Berl) 161:304–313
Carroll ME, Lynch WJ, Roth ME, Morgan AD (2004) Sex and estrogen influence drug abuse. Trends Pharmacol Sci 25:273–279
Carroll ME, Anderson MM, Morgan AD (2007) Higher locomotor response to cocaine in female (vs. male) rats selectively bred for high (HiS) and low (LoS) saccharin intake. Pharmacol Biochem Behav 88:94
Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK (2008) Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol 19:435–460
Carroll ME, Anker JJ, Perry JL (2009) Modeling risk factors for nicotine and other drug abuse in the preclinical laboratory. Drug Alcohol Depend 104:S70–S78
Chester JA, Barrenha GD (2007) Acoustic startle at baseline and during acute alcohol withdrawal in replicate mouse lines selectively bred for high or low alcohol preference. Alcohol Clin Exp Res 31:1633–1644
Chester JA, Blose AM, Froehlich JC (2003) Further evidence of an inverse genetic relationship between innate differences in alcohol preference and alcohol withdrawal magnitude in multiple selectively bred rat lines. Alcohol Clin Exp Res 27:377–387
Chester JA, Blose AM, Froehlich JC (2004) Acoustic startle reactivity during acute alcohol withdrawal in rats that differ in genetic predisposition toward alcohol drinking: effect of stimulus characteristics. Alcohol Clin Exp Res 28:677–687
Chester JA, Blose AM, Froehlich JC (2005) Effects of chronic alcohol treatment on acoustic startle reactivity during withdrawal and subsequent alcohol intake in high and low alcohol drinking rats. Alcohol Alcohol 40:379–387
Cicero TJ, Nock B, Meyer ER (2002) Gender-linked differences in the expression of physical dependence in the rat. Pharmacol Biochem Behav 72:691–697
National Research Council (2011) Guide for the care and use of laboratory animals, 8th edn. National Academies Press, Washington DC
Craft RM, Stratmann JA, Bartok RE, Walpole TI, King SJ (1999) Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacol (Berl) 143:1–7
Davis M (2006) Neural systems involved in fear and anxiety measured with fear-potentiated startle. Am Psychol 61:741–756
Deshmukh A, O’Reilly A, Pfefferbaum A, Rosenboom MJ, Sassoon S, Sullivan EV (2003) Alcoholic men endorse more DSM-IV withdrawal symptoms than alcoholic women matched in drinking history. J Stud Alcohol 64:375–379
Dess NK, Minor TR (1996) Taste and emotionality in rats selectively bred for high versus low saccharin intake. Anim Learn Behav 24:105–115
Dess NK, Badia-Elder NE, Thiele TE, Kiefer SW, Blizard DA (1998) Ethanol consumption in rats selectively bred for differential saccharin intake. Alcohol 16:275–278
Dess NK, Arnal J, Chapman CD, Siebel S, VanderWeele DA, Green KF (2000) Exploring adaptations to famine: rats selectively bred for differential intake of saccharin differ on deprivation-induced hyperactivity and emotionality. Int J Comp Psychol 13:34–52
Dess NK, O’Neill P, Chapman CD (2005) Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol 37:9–22
Devaud LL, Chadda R (2001) Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcohol Clin Exp Res 25:1689–1696
Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Danney JW, Belin D (2012) High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacol (Berl) 222:89–97
Engelmann JM, Radke AK, Gewirtz JC (2009) Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacol (Berl) 207:13–25
Fadel J, Deutch AY (2002) Anatomical substrates of orexin–dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience 111:379–387
Ferguson SG, Shiffman S, Gwaltney CJ (2006) Does reducing withdrawal severity mediate nicotine patch efficacy? A randomized clinical trial. J Consult Clin Psychol 74:1153–1161
Froehlich J, Harts J, Lumeng L, Li T (1988) Differences in response to the aversive properties of ethanol in rats selectively bred for oral ethanol preference. Pharmacol Biochem Behav 31:215–222
Gatch MB, Lal H (2001) Animal models of the anxiogenic effects of ethanol withdrawal. Drug Dev Res 54:95–115
Gellert VF, Holtzman SG (1978) Development and maintenance of morphine tolerance and dependence in the rat by scheduled access to morphine drinking solutions. J Pharmacol Exp Ther 205:536–546
Georgescu D, Zachariou V, Barrott M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ (2003) Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. J Neurosci 23:3106–3111
Gosnell BA, Levine AS (2009) Reward systems and food intake: role of opioids. Int J Obes (Lond) 33:S54–S58
Harris AC, Gewirtz JC (2004) Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacol (Berl) 171:140–147
Harris AC, Atkinson DM, Aase DM, Gewirtz JC (2006) Double dissociation in the neural substrates of acute opiate dependence as measured by withdrawal-potentiated startle. Neuroscience 139:1201–1210
Higgins GA, Sellers EM (1994) Antagonist-precipitated opioid withdrawal in rats: evidence for dissociations between physical and motivational signs. Pharmacol Biochem Behav 48:1–8
Holtz NA, Carroll ME (2011) Baclofen has opposite effects on escalation of cocaine self-administration: increased intake in rats selectively bred for high (HiS) saccharin intake and decreased intake in those selected for low (LoS) saccharin intake. Pharmacol Biochem Behav 100:275–283
Holtz NA, Zlebnik NE, Carroll ME (2012) Differential orexin/hypocretin expression in addiction-prone and -resistant rats selectively bred for high (HiS) and low (LoS) saccharin intake. Neurosci Lett 522:12–15
Janowsky D, Pucilowski O, Buyinza M (2003) Preference for higher sucrose concentrations in cocaine abusing-dependent patients. J Psychiatr Res 37:35–41
Kampman KM, Alterman AI, Volpicelli JR, Maany I, Muller ES, Luce DD, Mulholland EM, Jawad AF, Parikh GA, Mulvaney FD, Weinrieb RM, O’Brien CP (2001) Cocaine withdrawal symptoms and initial urine toxicology results predict treatment attrition in outpatient cocaine dependence treatment. Psychol Addict Behav 15:52–59
Kampov Polevoy AB, Garbutt JC, Janowsky D (1997) Evidence of preference for a high-concentration sucrose solution in alcoholic men. Am J Psychiatry 154:269–270
Kampov Polevoy AB, Tsoi MV, Zvartau EE, Neznanov NG, Khalitov E (2001) Sweet liking and family history of alcoholism in hospitalized alcoholic and non-alcoholic patients. Alcohol Alcohol 36:165–170
Kelsey JE, Arnold SR (1994) Lesions of the dorsomedial amygdala, but not the nucleus accumbens, reduce the aversiveness of morphine withdrawal in rats. Behav Neurosci 108:1119–1127
Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006) Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26:5894–5900
Kest B, Palmese CA, Hopkins E, Adler M, Juni A (2001) Assessment of acute and chronic morphine dependence in male and female mice. Pharmacol Biochem Behav 70:149–156
Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278:52–58
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–268
Koob GF, Maldonado R, Stinus L (1992) Neural substrates of opiate withdrawal. Trends Neurosci 15:186–191
Kosten TA, Ambrosio E (2002) HPA axis function and drug addictive behaviors: insights from studies with Lewis and Fischer 344 inbred rats. Psychoneuroendocrino 27:35–69
Lancellotti D, Bayer BM, Glowa JR, Houghtling RA, Riley AL (2001) Morphine-induced conditioned taste aversions in the LEW/N and F344/N rat strains. Pharmacol Biochem Behav 68:603–610
Laviolette SR, Nader K, van der Kooy D (2002) Motivational state determines the functional role of the mesolimbic dopamine system in the mediation of opiate reward processes. Behav Brain Res 129:17–29
Lohmiller JJ, Swing SP (2006) Reproduction and breeding. In: Suckow A, Weisbroth SH, Franklin CL (eds) The laboratory rat, 2nd edn. Academic, Burlington, pp 147–162
Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK (1998) High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome 9:983–990
Mysels DJ, Sullivan MA (2010) The relationship between opioid and sugar intake: review of evidence and clinical applications. J Opioid Manag 6:445–452
Nakagawa T, Yamamoto R, Fujio M, Suzuki Y, Minami M, Satoh M, Kaneko S (2005) Involvement of the bed nucleus of the stria terminalis activated by the central nucleus of the amygdala in the negative affective component of morphine withdrawal in rats. Neuroscience 134:9–19
Overstreet DH, Knapp DJ, Breese GR (2004) Similar anxiety-like responses in male and female rats exposed to repeated withdrawal from ethanol. Pharmacol Biochem Behav 78:459–464
Perry JL, Dess NK, Morgan AD, Carroll ME (2006a) Escalation of iv cocaine self-administration and reinstatement of cocaine-seeking behavior in rats bred for high and low saccharin intake. Psychopharmacol (Berl) 186:235–245
Perry JL, Normile LM, Morgan AD, Carroll ME (2006b) Sex differences in physical dependence on orally self-administered phencyclidine (PCP) in rhesus monkeys (Macaca mulatta). Exp Clin Psychopharmacol (Berl) 14:68–78
Pomerleau CS, Garcia AW, Drewnowski A, Pomerleau OF (1991) Sweet taste preference in women smokers: comparison with nonsmokers and effects of menstrual phase and nicotine abstinence. Pharmacol Biochem Behav 40:995–999
Radke AK, Gewirtz JC (2012) Increased dopamine receptor activity in the nucleus accumbens shell ameliorates anxiety during drug withdrawal. Neuropsychopharmacology 37:2405–2415
Radke AK, Rothwell PE, Gewirtz JC (2011) An anatomical basis for opponent process mechanisms of opiate withdrawal. J Neurosci 31:7533–7539
Rothwell PE, Thomas MJ, Gewirtz JC (2009) Distinct profiles of anxiety and dysphoria during spontaneous withdrawal from acute morphine exposure. Neuropsychopharmacology 34:2285–2295
Schramm-Sapyta NL, Morris RW, Kuhn CM (2006) Adolescent rats are protected from the conditioned aversive properties of cocaine and lithium chloride. Pharmacol Biochem Behav 84:344–352
Schulteis G, Heyser CJ, Koob GF (1997) Opiate withdrawal signs precipitated by naloxone following a single exposure to morphine: potentiation with a second morphine exposure. Psychopharmacol (Berl) 129:56–65
Schulteis G, Heyser CJ, Koob GF (1999) Differential expression of response-disruptive and somatic indices of opiate withdrawal during the initiation and development of opiate dependence. Behav Pharmacol 10:235–242
Schulteis G, Morse AC, Liu J (2003) Repeated experience with naloxone facilitates acute morphine withdrawal: potential role for conditioning processes in acute opioid dependence. Pharmacol Biochem Behav 76:493–503
Sharf R, Sarhan M, DiLeone RJ (2008) Orexin mediated the expression of precipitated morphine withdrawal and concurrent activation of the nucleus accumbens shell. Biol Psychiatry 64:175–183
Shram MJ, Funk D, Li Z, Le AD (2006) Periadolescent and adult rats respond differently in tests measuring the rewarding and aversive effects of nicotine. Psychopharmacol (Berl) 186:201–208
Smith RJ, Aston-Jones G (2008) Noradrenergic transmission in the extended amygdala: role in increased drug-seeking and relapse during protracted drug abstinence. Brain Struct Funct 213:43–61
Solomon RL, Corbit JD (1974) An opponent-process theory of motivation. I. Temporal dynamics of affect. Psychol Rev 81:119–145
Stewart R, McBride W, Lumeng L, Li TK, Murphy J (1991) Chronic alcohol consumption in alcohol-preferring P rats attenuates subsequent conditioned taste aversion produced by ethanol injections. Psychopharmacol (Berl) 105:530–534
Stewart RB, Murphy J, McBride WJ, Lumeng L, Li TK (1996) Place conditioning with alcohol in alcohol-preferring and-nonpreferring rats. Pharmacol Biochem Behav 53:487–491
Suzuki T, Koike Y, Yoshii T, Yanaura S (1985) Sex differences in the induction of physical dependence on pentobarbital in the rat. Jpn J Pharmacol 39:453–459
Suzuki T, Koike Y, Misawa M (1988) Sex differences in physical dependence on methaqualone in the rat. Pharmacol Biochem Behav 30:483–488
Suzuki T, Motegi H, Otani K, Koike Y, Misawa M (1992) Susceptibility to, tolerance to, and physical dependence on ethanol and barbital in two inbred strains of rats. Gen Pharmacol 23:11–17
Suzuki T, Ise Y, Maed J, Misawa M (1999) Mecamylamine-precipitated nicotine-withdrawal aversion in Lewis and Fischer 344 inbred rat strains. Eur J Pharmacol 369:159–162
Tzschentke TM (1998) Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol 56:613–672
Tzschentke TM (2007) Review on CPP: Measuring reward with the conditioned place preference (CPP) paradigm: update of the last decade. Addict Biol 12:227–462
Varlinskaya EI, Spear LP (2004) Acute ethanol withdrawal (hangover) and social behavior in adolescent and adult male and female Sprague–Dawley rats. Alcohol Clin Exp Res 28:40–50
Walker QD, Nelson CJ, Smith D, Kuhn CM (2002) Vaginal lavage attenuates cocaine-stimulated activity and establishes place preference in rats. Pharmacol Biochem Behav 73:743–752
Watanabe T, Nakagawa T, Yamamoto R, Maeda A, Minami M, Satoh M (2002a) Involvement of glutamate receptors within the central nucleus of the amygdala in naloxone-precipitated morphine withdrawal-induced conditioned place aversion in rats. Jpn J Pharmacol 88:399–406
Watanabe T, Yamamoto R, Maeda A, Nakagawa T, Minami M, Satoh M (2002b) Effects of excitotoxic lesions of the central or basolateral nucleus of the amygdala on naloxone-precipitated withdrawal-induced conditioned place aversion in morphine-dependent rats. Brain Res 958:423–428
Wee S, Koob GJ (2010) The role of the dynorphin–kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacol (Berl) 210:121–135
Weiss G (1982) Food fantasies of incarcerated drug users. Int J Addict 17:905–912
West RJ, Hajek P, Belcher M (1989) Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med 19:981–985
Wise RA, Bozarth MA (1987) A psychomotor stimulant theory of addiction. Psychol Rev 94:469–492
Zhou Y, Bendor J, Hofmann L, Randesi M, Ho A, Kreek MJ (2006) Mu opioid receptor and orexin/hypocretin mRNA levels in the lateral hypothalamus and striatum are enhanced by morphine withdrawal. J Endocrinol 191:137–145
Acknowledgments
We thank Sofiya Hupalo and Jacob Leslie for technical assistance, Gail Towers for animal husbandry, Dr. Mark Thomas for the use of the place-conditioning apparatus, and Dr. Andrew Harris for assistance measuring somatic withdrawal signs. This work was funded by NIDA grants K05 DA015267 and P20 DA024196 (MEC) and the University of Minnesota (JCG).
Conflict of interest
The authors declare no conflict of interest in regard to this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Radke, A.K., Holtz, N.A., Gewirtz, J.C. et al. Reduced emotional signs of opiate withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Psychopharmacology 227, 117–126 (2013). https://doi.org/10.1007/s00213-012-2945-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-012-2945-0