Abstract
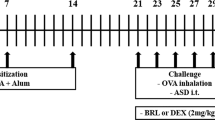
Allergic asthma is an immunological disease that occurs as a consequence of aeroallergen exposure. Inhibition of poly(ADP-ribose) polymerases (PARPs) in conventional models of asthma-like reaction has emerged as an effective anti-inflammatory and airway remodeling intervention. In a house dust mite (HDM) exposure mouse model, we investigated the impact of PARP inhibition on allergic airway inflammation, sensitization, and remodeling. Mice were intranasally exposed to a HDM extract for 5 days per week for up to 5 weeks. Mice were administered, or not, by PARP inhibitors 3-aminobenzamide (3-ABA) or 5-aminoisoquinolinone (5-AIQ) during the last 2 weeks of HDM treatment. Mice treated with PARP inhibitors after HDM stimulation showed a significant decrease in the number of total cells and eosinophils detectable in the bronchoalveolar lavage fluid as compared with the HDM-stimulated ones. In vitro HDM-stimulated splenocyte culture produced considerable amounts of the Th2 cytokines that were not affected by treatment with PARP inhibitors. Immunoglobulin levels in the serum were also unchanged. In the lung tissue, collagen deposition was decreased, whereas α-smooth muscle actin thickening was not significantly affected. Moreover, in HDM-stimulated PARP inhibitor-treated groups, we found a downregulation in the activation of signal transducer and activator of trascription-6 (STAT-6) and a significant decrease in the mRNA levels of C-C motif chemokine 11 (CCL11). In this mouse model of chronic asthma PARP inhibition treatment, although it does not affect sensitization, it effectively reduces the allergic airway inflammation and affects the remodeling through a mechanism involving STAT6 and CCL11.







Similar content being viewed by others
References
Barnes PJ (2004) New drugs for asthma. Nat Rev Drug Discov 3:831–844
Barnes PJ (2006) Transcription factors in airway diseases. Lab Investig 86:867–872
Boulares AH, Zoltoski AJ, Sherif ZA, Jolly P, Massaro D, Smulson ME (2003) Gene knockout or pharmacological inhibition of poly(ADP-ribose) polymerase-1 prevents lung inflammation in a murine model of asthma. Am J Respir Cell Mol Biol 28:322–329
Bousquet J, Clark TJ, Hurd S, Khaltaev N, Lenfant C, O’Byrne P (2007) GINA guidelines on asthma and beyond. Allergy 62:102–112
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bürkle A (2005) Poly(ADP-ribose). The most elaborate metabolite of NAD+. FEBS J 272:4576–4589
Cates EC, Fattouh R, Wattie J, Inman MD, Goncharova S, Coyle AJ, Gutierrez-Ramos JC, Jordana M (2004) Intranasal exposure of mice to house dust mite elicits allergic airway inflammation via a GM-CSF-mediated mechanism. J Immunol 173:6384–6392
Cates EC, Fattouh R, Johnson JR, Llop-Guevara A, Jordana M (2007) Modeling responses to respiratry house dust mite exposure. Models of exacerbation of asthma and COPD. Contrib Microbiol 14:42–67
Chomczynsky P, Sacchi N (1987) Single step method of RNA isolation by acid guanidinium thiocynate-phenol-chloroform extraction. Anal Biochem 162:156–159
Cuzzocrea S (2005) Shock, inflammation and PARP. Pharmacol Res 52:72–82
Cuzzocrea S, Mc Donald MC, Mazzon E, Dugo L, Serraino I, Threadgill M, Caputi AP, Thiemermann C (2002) Effect of 5-amminoisoquinolinone, a water-soluble, potent inhibitor of the activity of poly(ADP-ribose) polymerase, in a rodent model of lung injury. Biochem Pharmacol 63:293–304
D’Amours D, Desnoyers S, D’Silva I, Poirer GG (1999) Poly(ADP-rybosil)ation reactions in the regulation of nuclear functions. Biochem J 342:249–268
Fahy JV (2001) Remodelling of the airway epithelium in asthma. Am. J. Respir. Crit Care Med 164:S46–S51
Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, Coyle AJ, Humbles AA, Jordana M (2011) Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease. Am J Respir Crit Care Med 183:179–188
Fulkerson PC, Zimmermann N, Hassman LM, Finkelman FD, Rothenberg ME (2004) Pulmonary chemokine expression is coordinately regulated by STAT-1, STAT-6 and IFN-gamma. J Immunol 173:7565–7574
Hammad H, Lambrecht BN (2006) Recent progress in the biology of airway dendritic cells and implications for understanding the regulation of asthmatic inflammation. J Allergy Clin Immunol 118:331–336
Hebenstreit D, Wirnsberger G, Horejs-Hoeck J, Duschl A (2006) Signalling mechanisms, interaction partners, and target genes of STAT-6. Cytokine Growth Factors Rev 17:173–188
Hoeck J, Woisetschlager M (2001) STAT-6 mediates eotaxin-1 expression in IL-4 or TNF-alpha-induced fibroblasts. J Immunol 166:4507–4515
Holgate ST (2002) Airway inflammation and remodelling in asthma: current concepts. Mol Biotechnol 22:179–189
Jagtap P, Szàbo C (2005) Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat Rev Drug Discov 4:421–440
Johnson RJ, Wiley ER, Fattouh R, Swirski KF, Gajewska UB, Coyle JA, Gutierrez-Ramos JC, Ellis R, Inman DM, Jordana M (2004) Continuous exposure to house dust mite elicits chronic airway inflammation and structural remodeling. Am J Respir Crit Care Med 169:378–385
Kay AB (2005) The role of eosinophils in the pathogenesis of asthma. Trends Mol Med 11:148–152
Kuperman D, Schofield B, Wills-Karp M, Grusby MJ (1998) Signal transducer and activator of transcription 6 (STAT6)-deficient mice are protected from antigen-induced airway hyperresponsiveness and mucus production. J Exp Med 187:939–948
Kurucz I, Szeleny I (2006) Current animal models of bronchial asthma. Curr Pharm Des 12:3175–3194
Lucarini L, Pini A, Gerace E, Pellicciari R, Masini E, Moroni F (2014) Poly(ADP-ribose) polymerase inhibition with HYDAMTIQ reduces allergen-induced asthma-like reaction, bronchial hyper-reactivity and airway remodeling. J Cell Mol Med 18:468–479
Lukacs NW, Oliveira SHP, Hogaboam CM (1999) Chemokines and asthma: redundancy of function or a coordinated effort? J Clin Invest 104:995–999
MacNee W (2001) Oxidative stress and lung inflammation airways disease. Eur J Pharmacol 429:195–207
Masoli M, Fabian D, Holt S, Beasley R (2004) Global initiative for asthma (GINA) program: the global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 59:469–478
Mehrotra P, Hollenbeck A, Riley JP, Li F, Patel RJ, Akhtar N, Goenka S (2013) Poly (ADP-ribose)polymerase 14 and its enzyme activity regulates T(H)2 differentiation and allergic airway disease. J Allergy Clin Immunol 131:521–531
Menegazzi M, Di Paola R, Mazzon E, Genovese T, Crisafulli C, Dal Bosco M, Zou Z, Suzuki H, Cuzzocrea S (2008) Glycyrrhizin attenuates the development of carrageenan-induced lung injury in mice. Pharmacol Res 58:22–31
Naura AS, Hans CP, Zerfaoui M, You D, Cormier SA, Oumouna M, Boulares AH (2008) Post-allergen challenge inhibition of poly(ADP-ribose) polymerase harbors therapeutic potential for treatment of allergic airway inflammation. Clin Exp Allergy 38:839–846
Ngoc LP, Gold DR, Tzianabos AO, Weiss ST, Celedon JC (2005) Cytokines, allergy and asthma. Curr Opin Allergy Clin Immunol 5:161–166
Oumouna M, Datta R, Oumouna-Benachour K, Suzuki Y, Hans C, Matthews K, Fallon K, Boulares H (2006) Poly(ADP-ribose) polymerase-1inhibition prevents eosinophils recruitment by modulating Th2 cytokines in a murine model of allergic airway inflammation: a potential specific effect on IL-5. J Immunol 177:6489–6496
Pease JE, Williams TJ (2001) Eotaxin and asthma. Curr Opin Pharmacol 1:248–253
Robinson CB, Leonard J, Panettieri RA Jr (2012) Drug development for severe asthma: what are the metrics? Pharmacol There 135:176–118
Sampath D, Castro M, Look DC, Holtzman MJ (1999) Constitutive activation of an epithelial signal transducer and activator of transcription (STAT) pathway in asthma. J Clin Invest 103:1353–1361
Singh AM, Busse WW (2006) Asthma exacerbation 2: aetiology. Thorax 61:809–816
Stampfli MR, Wiley RE, Neigh GS, Gajewska BU, Lei XF, Snider DP, Xing Z, Jordana M (1998) GM-CSF transgene expression in the airway allows aerosolized ovalbumin to induce allergic sensitization in mice. J Clin Inves 102:1704–1714
Steffen JD, Brody JR, Armen RS, Pascal JM (2013) Structural implications for selective targeting of PARPs. Front Oncol doi. doi:10.3389/fonc.2013.00301
Suzuki Y, Masini E, Mazzocca C, Cuzzocrea S, Ciampa A, Suzuki H, Bani D (2004) Inhibition of poly(ADP-ribose) polymerase prevents allergen-induced asthma-like reaction in sensitized Guinea pigs. J Pharmacol Exp Therapy 311:1241–1248
Virag L, Bay P, Bak I, Pacher P, Mabley JD, Liaudet L, Bakondi E, Gergely P, Kollai M, Szabo C (2004) Effects of poly(ADP-ribose) polymerase inhibition on inflammatory cell migration in a murine model of asthma. Med Sci Monit 10:BR77–BR83
Yu SW, Wang H, Poitras MF, Coombs C, Bowers WJ, Federoff HJ, Poirier GG, Dawson TM, Dawson VL (2002) Mediation of poly(ADP-ribose) polymerase −1-dependent cell death by apoptosis-inducing factor. Science 297:259–263
Zock JP, Heinrich J, Jarvis D, Verlato G, Norback D, Plana E, Sunyer J, Chinn S, Olivieri M, Soon A, Villani S, Ponzio M, Dahlmann-Hoglund A, Svanes C, Luczynska C (2006) Distribution and determinants of house dust mite allergens in Europe: the European Community respiratory health survey II. J Allergy Clin Immunol 118:682–690
Acknowledgments
The authors are grateful to Ramzi Fattouh for technical assistance and to Manel Jordana that allows Raffaela Zaffini to join his laboratory: Dep. of Pathology and Molecular Medicine and Division of Respiratory Diseases and Allergy, Center of Gene Therapeutics, MDCL, McMaster University, Hamilton, Ontario, Canada. Part of the results reported in the manuscript has been presented by RZ at the XIX National Congress of Poly-ADP Ribosylation Reaction.
This work was supported by Fondo Unico per la Ricerca (FUR)/Menegazzi, Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR), Rome, Italy.
This paper is dedicated to the memory of Hisanori Suzuki, who passed away on 19 March 2012 due to a rapidly overwhelming disease. We all remember our friend as a master in life and science, and we hope that he will continue to help us from the place where he is now.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Zaffini, R., Di Paola, R., Cuzzocrea, S. et al. PARP inhibition treatment in a nonconventional experimental mouse model of chronic asthma. Naunyn-Schmiedeberg's Arch Pharmacol 389, 1301–1313 (2016). https://doi.org/10.1007/s00210-016-1294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-016-1294-7