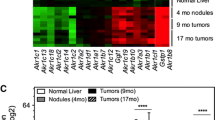
Abstract
Genotoxic carcinogens pose great hazard to human health. Uncertainty of current risk assessment strategies and long latency periods between first carcinogen exposure and diagnosis of tumors have raised interest in predictive biomarkers. Initial DNA adduct formation is a necessary step for genotoxin induced carcinogenesis. However, as DNA adducts not always translate into tumorigenesis, their predictive value is limited. Here we hypothesize that the combined analysis of pro-mutagenic DNA adducts along with time-matched gene expression changes could serve as a superior prediction tool for genotoxic carcinogenesis. Eker rats, heterozygous for the tuberous sclerosis (Tsc2) tumor suppressor gene and thus highly susceptible towards genotoxic renal carcinogens, were continuously treated with the DNA alkylating carcinogen methylazoxymethanol acetate (MAMAc). Two weeks of MAMAc treatment resulted in a time-dependent increase of O6-methylguanine and N7-methylguanine adducts in the kidney cortex, which was however not reflected by significant expression changes of cyto-protective genes involved in DNA repair, cell cycle arrest or apoptosis. Instead, we found a transcriptional regulation of genes involved in the tumor-related MAPK, FoxO and TGF-beta pathways. Continuous MAMAc treatment for up to 6 months resulted in a mild but significant increase of cancerous lesions. In summary, the combined analysis of DNA adducts and early gene expression changes could serve as a suitable predictive tool for genotoxicant-induced carcinogenesis.





Similar content being viewed by others
References
Ashburner M, Ball CA, Blake JA et al (2000) Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25(1):25–29. doi:10.1038/75556
Beranek DT (1990) Distribution of methyl and ethyl adducts following alkylation with monofunctional alkylating agents. Mutat Res 231(1):11–30
Bielas JH, Heddle JA (2000) Proliferation is necessary for both repair and mutation in transgenic mouse cells. Proc Natl Acad Sci USA 97(21):11391–11396. doi:10.1073/pnas.190330997
Boysen G, Pachkowski BF, Nakamura J, Swenberg JA (2009) The formation and biological significance of N7-guanine adducts. Mutat Res 678(2):76–94. doi:10.1016/j.mrgentox.2009.05.006
Clewell RA, Sun B, Adeleye Y et al (2014) Profiling dose-dependent activation of p53-mediated signaling pathways by chemicals with distinct mechanisms of DNA damage. Toxicol Sci 142(1):56–73. doi:10.1093/toxsci/kfu153
Cohen SM, Ellwein LB (1990) Cell proliferation in carcinogenesis. Science 249(4972):1007–1011
Coulondre C, Miller JH (1977) Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol 117(3):577–606
Dietrich DR, Swenberg JA (1991) Preneoplastic lesions in rodent kidney induced spontaneously or by non-genotoxic agents: predictive nature and comparison to lesions induced by genotoxic carcinogens. Mutat Res 248(2):239–260
Ellinger-Ziegelbauer H, Stuart B, Wahle B, Bomann W, Ahr HJ (2004) Characteristic expression profiles induced by genotoxic carcinogens in rat liver. Toxicol Sci 77(1):19–34. doi:10.1093/toxsci/kfh016
Ellinger-Ziegelbauer H, Stuart B, Wahle B, Bomann W, Ahr HJ (2005) Comparison of the expression profiles induced by genotoxic and nongenotoxic carcinogens in rat liver. Mutat Res 575(1–2):61–84. doi:10.1016/j.mrfmmm.2005.02.004
Fahrer J, Frisch J, Nagel G et al (2015) DNA repair by MGMT, but not AAG, causes a threshold in alkylation-induced colorectal carcinogenesis. Carcinogenesis 36(10):1235–1244. doi:10.1093/carcin/bgv114
Fu D, Calvo JA, Samson LD (2012) Balancing repair and tolerance of DNA damage caused by alkylating agents. Nat Rev Cancer 12(2):104–120. doi:10.1038/nrc3185
Gene Ontology C (2015) Gene Ontology Consortium: going forward. Nucleic Acids Res 43(Database issue):D1049–D1056. doi:10.1093/nar/gku1179
Guerard M, Baum M, Bitsch A et al (2015) Assessment of mechanisms driving non-linear dose-response relationships in genotoxicity testing. Mutat Res Rev Mutat Res 763:181–201. doi:10.1016/j.mrrev.2014.11.001
Gusek W, Mestwerdt W (1969) Cycasin-induced renal tumors in the Wistar rat with special consideration of the adenoma. Beitr Pathol Anat 139(2):199–218
Habib SL (2010) Tuberous sclerosis complex and DNA repair. Adv Exp Med Biol 685:84–94
Jarabek AM, Pottenger LH, Andrews LS et al (2009) Creating context for the use of DNA adduct data in cancer risk assessment: I. Data organization. Crit Rev Toxicol 39(8):659–678. doi:10.1080/10408440903164155
Jensen LJ, Kuhn M, Stark M et al (2009) STRING 8–a global view on proteins and their functional interactions in 630 organisms. Nucleic Acids Res 37(Database issue):D412–D416. doi:10.1093/nar/gkn760
Kisby GE, Fry RC, Lasarev MR et al (2011) The cycad genotoxin MAM modulates brain cellular pathways involved in neurodegenerative disease and cancer in a DNA damage-linked manner. PLoS One 6(6):e20911. doi:10.1371/journal.pone.0020911
Laqueur GL, McDaniel EG, Matsumoto H (1967) Tumor induction in germfree rats with methylazoxymethanol (MAM) and synthetic MAM acetate. J Natl Cancer Inst 39(2):355–371
Margison GP, Santibanez Koref MF, Povey AC (2002) Mechanisms of carcinogenicity/chemotherapy by O6-methylguanine. Mutagenesis 17(6):483–487
Matsubara N, Mori H, Hirono I (1978) Effect of colostomy on intestinal carcinogenesis by methylazoxymethanol acetate in rats. J Natl Cancer Inst 61(4):1161–1164
Matsumoto H, Higa HH (1966) Studies on methylazoxymethanol, the aglycone of cycasin: methylation of nucleic acids in vitro. Biochem J 98(2):20 C-22 C
Matsushima T, Matsumoto H, Shirai A, Sawamura M, Sugimura T (1979) Mutagenicity of the naturally occurring carcinogen cycasin and synthetic methylazoxymethanol conjugates in Salmonella typhimurium. Cancer Res 39(9):3780–3782
McDorman KS, Hooth MJ, Starr TB, Wolf DC (2003) Analysis of preneoplastic and neoplastic renal lesions in Tsc2 mutant Long-Evans (Eker) rats following exposure to a mixture of drinking water disinfection by-products. Toxicology 187(1):1–12
Morton LD, Youssef AF, Lloyd E, Kiorpes AL, Goldsworthy TL, Fort FL (2002) Evaluation of carcinogenic responses in the Eker rat following short-term exposure to selected nephrotoxins and carcinogens. Toxicol Pathol 30(5):559–564
Notman J, Tan QH, Zedeck MS (1982) Inhibition of methylazoxymethanol-induced intestinal tumors in the rat by pyrazole with paradoxical effects on skin and kidney. Cancer Res 42(5):1774–1780
OECD (1981) OECD Guidelines for the Testing of Chemicals. Section 4. Health effects
Patel SK, Ma N, Monks TJ, Lau SS (2003) Changes in gene expression during chemical-induced nephrocarcinogenicity in the Eker rat. Mol Carcinog 38(3):141–154. doi:10.1002/mc.10153
Preston-Martin S, Pike MC, Ross RK, Jones PA, Henderson BE (1990) Increased cell division as a cause of human cancer. Cancer Res 50(23):7415–7421
Sakumi K, Shiraishi A, Shimizu S, Tsuzuki T, Ishikawa T, Sekiguchi M (1997) Methylnitrosourea-induced tumorigenesis in MGMT gene knockout mice. Cancer Res 57(12):2415–2418
Sharma V, Collins LB, Clement JM, Zhang Z, Nakamura J, Swenberg JA (2014) Molecular dosimetry of endogenous and exogenous O(6)-methyl-dG and N7-methyl-G adducts following low dose [D3]-methylnitrosourea exposures in cultured human cells. Chem Res Toxicol 27(4):480–482. doi:10.1021/tx5000602
Smyth GK (2005) Limma: linear models for microarray data. In: Gentleman R, Carey V, Dudoit S, Irizarry R, Huber W (eds) Bioinformatics and computational biology solutions using R and bioconductor. Springer, New York, pp 397–420
Sohn OS, Fiala ES, Requeijo SP, Weisburger JH, Gonzalez FJ (2001) Differential effects of CYP2E1 status on the metabolic activation of the colon carcinogens azoxymethane and methylazoxymethanol. Cancer Res 61(23):8435–8440
Srivenugopal KS, Yuan XH, Friedman HS, Ali-Osman F (1996) Ubiquitination-dependent proteolysis of O6-methylguanine-DNA methyltransferase in human and murine tumor cells following inactivation with O6-benzylguanine or 1,3-bis(2-chloroethyl)-1-nitrosourea. Biochemistry 35(4):1328–1334. doi:10.1021/bi9518205
Stemmer K, Ellinger-Ziegelbauer H, Ahr HJ, Dietrich DR (2007) Carcinogen-specific gene expression profiles in short-term treated Eker and wild-type rats indicative of pathways involved in renal tumorigenesis. Cancer Res 67(9):4052–4068. doi:10.1158/0008-5472.CAN-06-3587
Stemmer K, Ellinger-Ziegelbauer H, Ahr HJ, Dietrich DR (2009) Molecular characterization of preneoplastic lesions provides insight on the development of renal tumors. Am J Pathol 175(4):1686–1698. doi:10.2353/ajpath.2009.081071
Swann PF (1990) Why do O6-alkylguanine and O4-alkylthymine miscode? The relationship between the structure of DNA containing O6-alkylguanine and O4-alkylthymine and the mutagenic properties of these bases. Mutat Res 233(1–2):81–94
Swenberg JA, Fryar-Tita E, Jeong YC et al (2008) Biomarkers in toxicology and risk assessment: informing critical dose-response relationships. Chem Res Toxicol 21(1):253–265. doi:10.1021/tx700408t
Swenberg JA, Lu K, Moeller BC et al (2011) Endogenous versus exogenous DNA adducts: their role in carcinogenesis, epidemiology, and risk assessment. Toxicol Sci 120(Suppl 1):S130–S145. doi:10.1093/toxsci/kfq371
Walker C, Goldsworthy TL, Wolf DC, Everitt J (1992) Predisposition to renal cell carcinoma due to alteration of a cancer susceptibility gene. Science 255(5052):1693–1695
Wang Y, Hu Z, Liu Z et al (2013) MTOR inhibition attenuates DNA damage and apoptosis through autophagy-mediated suppression of CREB1. Autophagy 9(12):2069–2086. doi:10.4161/auto.26447
Williams GM, Iatropoulos MJ, Wang CX et al (1996) Diethylnitrosamine exposure-responses for DNA damage, centrilobular cytotoxicity, cell proliferation and carcinogenesis in rat liver exhibit some non-linearities. Carcinogenesis 17(10):2253–2258
Yeung RS, Xiao GH, Jin F, Lee WC, Testa JR, Knudson AG (1994) Predisposition to renal carcinoma in the Eker rat is determined by germ-line mutation of the tuberous sclerosis 2 (TSC2) gene. Proc Natl Acad Sci USA 91(24):11413–11416
Yu G, Wang LG, Han Y, He QY (2012) clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16(5):284–287. doi:10.1089/omi.2011.0118
Acknowledgements
The authors thank Tanja Lampertsdoerfer, Gudrun von Scheven, Evelyn O’Brien and Alexandra Heussner for their skillful assistance during the animal experiment and Paul Pfluger for critically reading the manuscript. This work was supported by the Federal Ministry of Education and Research (BMBF: 0313024). The UNC Biomarker Mass Spectrometry Facility is partially supported by NIEHS grant P30-ES010126.
Author information
Authors and Affiliations
Corresponding author
Additional information
V. Klaus and H. Bastek contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Klaus, V., Bastek, H., Damme, K. et al. Time-matched analysis of DNA adduct formation and early gene expression as predictive tool for renal carcinogenesis in methylazoxymethanol acetate treated Eker rats. Arch Toxicol 91, 3427–3438 (2017). https://doi.org/10.1007/s00204-017-1953-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1953-6