Abstract
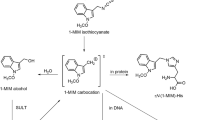
3-Nitrobenzanthrone (3-NBA), a potent environmental mutagen and carcinogen, is known to be activated in vivo to 3-benzanthronylnitrenium ion which forms both NH and C2-bound adducts with DNA and also reacts with glutathione giving rise to urinary 3-aminobenzanthron-2-ylmercapturic acid. In this study, acid hydrolysate of globin from rats dosed intraperitoneally with 3-NBA was analysed by HPLC/MS to identify a novel type of cysteine adduct, 3-aminobenzanthron-2-ylcysteine (3-ABA-Cys), confirmed using a synthesised standard. The 3-ABA-Cys levels in globin peaked after single 3-NBA doses of 1 and 2 mg/kg on day 2 to attain 0.25 and 0.49 nmol/g globin, respectively, thereafter declining slowly to 70–80% of their maximum values during 15 days. After dosing rats for three consecutive days with 1 mg 3-NBA/kg a significant cumulation of 3-ABA-Cys in globin was observed. 3-ABA-Cys was also found in the plasma hydrolysate. Herein, after dosing with 1 and 2 mg 3-NBA/kg the adduct levels peaked on day 1 at 0.15 and 0.51 nmol/ml plasma, respectively, thereafter declining rapidly to undetectable levels on day 15. In addition, sulphinamide adducts were also found in the exposed rats, measured indirectly as 3-aminobenzanthrone (3-ABA) split off from globin by mild acid hydrolysis. Levels of both types of adducts in the globin samples parallelled very well with 3-ABA/3-ABA-Cys ratio being around 1:8. In conclusion, 3-ABA-Cys is the first example of arylnitrenium-cysteine adduct in globin representing a new promising class of biomarkers to assess cumulative exposures to aromatic amines, nitroaromatics and heteroaromatic amines.






Similar content being viewed by others
Change history
20 May 2017
An erratum to this article has been published.
References
Arlt VM (2005) 3-Nitrobenzanthrone, a potential human cancer hazard in diesel exhaust and urban air pollution: review of the evidence. Mutagenesis 20:399–410. doi:10.1093/mutage/gei057
Arlt VM, Glatt H, Muckel E, Pabel U, Sorg BL, Seidel A, Frank H, Schmeiser HH, Phillips DH (2003a) Activation of 3-nitrobenzanthrone and its metabolites by human acetyltransferases, sulfotransferases and cytochrome P450 expressed in Chinese hamster V79 cells. Int J Cancer 105:583–592. doi:10.1002/ijc.11143
Arlt VM, Stiborová M, Hewer A, Schmeiser HH, Phillips DH (2003b) Human enzymes involved in the metabolic activation of the environmental contaminant 3-nitrobenzanthrone: evidence for reductive activation by human NADPH:cytochrome P450 reductase. Cancer Res 63:2752–2761
Arlt VM, Stiborová M, Henderson CJ, Osborne MR, Bieler CA, Frei E, Martínek V, Sopko B, Wolf CR, Schmeiser HH, Phillips DH (2005) The environmental pollutant and potent mutagen 3-nitrobenzanthrone forms DNA adducts after reduction by NAD(P)H:quinone oxidoreductase and conjugation by acetyltransferases and sulfotransferases in human hepatic cytosols. Cancer Res 65:2644–2652. doi:10.1158/0008-5472.CAN-04-3544
Arlt VM, Schmeiser HH, Osborne MR, Kawanishi M, Kanno T, Yagi T, Phillips DH, Takamura-Enya T (2006) Identification of three major DNA adducts formed by the carcinogenic air pollutant 3-nitrobenzanthrone in rat lung at the C8 and N2 position of guanine and at the N6 position of adenine. Int J Cancer 118:2139–2146. doi:10.1002/ijc.21622
Barber BJ, Schultz TJ, Randlett DL (1990) Comparative analysis of protein content in rat mesenteric tissue, peritoneal fluid, and plasma. Am J Physiol 258:G714–G718
Berlin NI, Berk PD (1975) The biological life of the red cell. In: Surgenor DM (ed) The red blood cell, 2nd edn, vol 2. Academic Press, New York, pp 957–1019
Boyland E, Manson D, Nery R (1963) Mercapturic acids as metabolites of aniline and 2-naphthylamine. Biochem J 86:263–271
Enya T, Suzuki H, Watanabe T, Hirayama T, Hisamatsu Y (1997) 3-Nitrobenzanthrone, a powerful bacterial mutagen and suspected human carcinogen found in diesel exhausts and airborne particulates. Environ Sci Technol 31:2772–2776. doi:10.1021/es961067i
Eyer P (1994) Reactions of oxidatively activated arylamines with thiols: reaction mechanisms and biological implications. An overview. Environ Health Perspect 102(Suppl. 6):123–132
Galleman D, Eyer P (1994) Additional pathways of S-conjugate formation during the interaction of thiols with nitrosoarenes bearing π-donating substituents. Environ Health Perspect 102(Suppl. 6):137–142
Jeffay H, Winzler RJ (1958) The effect of dietary protein on the turnover of rat serum protein. J Biol Chem 231:111–116
Kazanis S, McClelland RA (1992) Electrophilic intermediate in the reaction of glutathione and nitrosoarenes. J Am Chem Soc 114:3052–3059. doi:10.1021/ja00034a043
Kelava T, Ćavar I, Čulo F (2011) Biological actions of drug solvents. Period Biol 113(ISSN: 0031–5362):311–320
Linhart I, Mráz J, Hanzlíková I, Šilhánková A, Frantík E, Himl M (2012) Carcinogenic 3-nitrobenzanthrone but not 2-nitrobenzanthrone is metabolised to an unusual mercapturic acid in rats. Toxicol Lett 208:246–253. doi:10.1016/j.toxlet.2011.11.017
Morgan EH (1966) Transferrin and albumin distribution and turnover in the rat. Am J Physiol 211:1486–1494
Mráz J, Hanzlíková I, Moulisová A, Dušková Š, Hejl K, Bednářová A, Dabrowská L, Linhart I (2016) Hydrolytic cleavage products of globin adducts in urine as possible biomarkers of cumulative dose. Proof of concept using styrene oxide as a model adduct-forming compound. Chem Res Toxicol 29:676–686. doi:10.1021/acs.chemrestox.5b00518
Mulder GJ, Kadlubar FF, Mayes JB, Hinson JA (1984) Reaction of mutagenic phenacetin metabolites with glutathione and DNA. Possible implications for toxicity. Mol Pharmacol 26:342–347
Nagy E, Zeisig M, Kawamura K, Hisamatsu Y, Sugeta A, Adachi S, Moller L (2005) DNA adduct and tumor formations in rats after intratracheal administration of the urban air pollutant 3-nitrobenzanthrone. Carcinogenesis 26:1821–1828. doi:10.1093/carcin/bgi141
Neumann H-G, van Dorp C, Zwirner-Baier I (1995) The implications for risk assessment of measuring the relative contribution to exposure from occupation, environment and lifestyle: hemoglobin adducts from amino- and nitro-arenes. Toxicol Lett 82/83:771–778
Novak M, Chakraborti M (2011) N-Arylhydroxylamines and chemical carcinogenicity. In: Rappoport Z, Liebman JF (eds) The chemistry of hydroxylamines, oximes and hydroxamic acids, vol 2. John Wiley & Sons, New York, pp 577–621
Osborne MR, Arlt VM, Kliem C, Hull WE, Mirza A, Bieler CA, Schmeiser HH, Phillips DH (2005) Synthesis, characterization, and 32P-postlabeling analysis of DNA adducts derived from the environmental contaminant 3-nitrobenzanthrone. Chem Res Toxicol 18:1056–1070. doi:10.1021/tx0500474
Peng L, Turesky RJ (2013) Capturing labile sulfenamide and sulfinamide serum albumin adducts of carcinogenic arylamines by chemical oxidation. Anal Chem 85:1065–1072. doi:10.1021/ac3028273
Sabbioni G (1994) Hemoglobin binding of arylamines and nitroarenes: molecular dosimetry and quantitative structure-activity relationships. Environ Health Perspect 102(Suppl. 6):61–67
Safronov AI, Traven VF (1993) Synthesis of some imidazobenzanthrones. Zh Org Khim 29:1853–1958 (in Russian)
Stiborová M, Dračínská H, Hájková J, Kadeřábková P, Frei E, Schmeiser HH, Souček P, Phillips DH, Arlt VM (2006) The environmental pollutant and carcinogen 3-nitrobenzanthrone and its human metabolite 3-aminobenzanthrone are potent inducers of rat hepatic cytochromes P450 1A1 and-1A2 and NAD(P)H:quinone oxidoreductase. Drug Metab Dispos 34:1398–1405. doi:10.1124/dmd.106.009373
Stiborová M, Martínek V, Svobodová M, Šístková J, Dvořák Z, Ulrichová J, Šimánek V, Frei E, Schmeiser HH, Phillips DH, Arlt VM (2010) Mechanisms of the different DNA adduct forming potentials of the urban air pollutants 2-nitrobenzanthrone and carcinogenic 3-nitrobenzanthrone. Chem Res Toxicol 23:1192–1201. doi:10.1021/tx100052d
Straube E, Völkel W, Bringmann G, Dekant W (2005) Reaction of nitroso derivatives of dinitropyrenes with sulfhydryl groups of peptides and hemoglobin in vitro and in rats. Xenobiotica 35:1147–1164. doi:10.1080/00498250500342605
Takamura-Enya T, Kawanishi M, Takashi Y, Hisamatsu Y (2007) Structural identification of DNA adducts derived from 3-nitrobenzanthrone, a potent carcinogen present in the atmosphere. Chem Asian J 2:1174–1185. doi:10.1002/asia.200700061
Törnqvist M, Fred C, Haglund J, Helleberg H, Paulsson B, Rydberg P (2002) Protein adducts: quantitative and qualitative aspects of their formation, analysis and applications. J Chromatogr B 778:279–308
Acknowledgements
The study was supported by Ministry of Health of the Czech Republic-DRO (National Institute of Public Health-NIPH, IN 75010330) and University of Chemistry and Technology, Prague, Program of long-term development. Purchase of the principal analytical instrument, Orbitrap Q Exactive mass spectrometer, was supported by European Regional Development Fund (ERDF), Integrated Operational Programme Reg. No. CZ.1.06/3.2.01/11.08435. Skilled technical assistance of M. Tvrdíková, L. Dabrowská, H. Chrástecká, and R. Vajtrová is greatly appreciated.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s00204-017-1991-0.
Rights and permissions
About this article
Cite this article
Linhart, I., Hanzlíková, I., Mráz, J. et al. S-(3-Aminobenzanthron-2-yl)cysteine in the globin of rats as a novel type of adduct and possible biomarker of exposure to 3-nitrobenzanthrone, a potent environmental carcinogen. Arch Toxicol 91, 3317–3325 (2017). https://doi.org/10.1007/s00204-017-1943-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-017-1943-8