Abstract
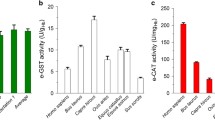
Liver and kidney glutathione S-transferase (GST) activities to 1,2-dichloro-4-nitrobenzene (DCNB) as a substrate (GST-D activities) were measured in 280 dogs from five different breeders, and significant individual differences in this activity were observed in both organs. Interestingly, 34 out of the 280 dogs (i.e. 12.1%) were those in which liver GST-D activities were less than 10 nmol/min per mg cytosolic protein, “low GST dogs”, and the other dogs were classified as “middle” and “high” GST dogs for which the liver GST-D activities were 10–80 and >80 nmol/min per mg protein, respectively, and occurred at similar percentages (41.4% for the middle GST dog and 46.4% for the high GST dog). Furthermore, the existence of the low GST dogs was not limited to one particular breeder. There was a good correlation (r=0.910) between the liver and kidney GST-D activities, showing low activity in not only the liver but also the kidney in the low GST dogs. Although liver GST activity to 1-chloro-2,4-dinitrobenzene as a substrate (GST-C activity), catalyzed by various GST isozymes in dogs, was significantly correlated with liver GST-D activity, GST-C activity showed more than 450 nmol/min per mg protein even in the low GST dogs. There was no significant difference in cytochrome P450 content, 7-ethoxycoumarin O-deethylase activity or UDP-glucuronosyltransferase activity to p-nitrophenol as a substrate between low GST dogs and the other dogs. Finally, remarkably high plasma concentrations of DCNB were observed in the low GST dogs after single doses of DCNB at 5 or 100 mg/kg. The individual differences in GST-D activity are probably attributable to the content and/or activity of the theta class GST isozyme YdfYdf since it has been reported that glutathione conjugation of DCNB is specifically catalyzed by GSTYdfYdf in dogs. In conclusion, we identified a number of low GST dogs in which the GST-D activities were not observed either in vivo or in vitro. The feasibility of using a single low dose of DCNB to phenotype dogs based on GST-D activity was confirmed. It was also suggested that low GST dogs have high susceptibility, including unexpected toxicity or abnormal exposure, to chemicals metabolized by GSTYdfYdf.







Similar content being viewed by others
References
Ali-Osman F, Akande O, Antoun G, Mao JX, Buolamwini J (1997) Molecular cloning, characterization and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants. Evidence for differential catalytic activity of the encoded proteins. J Biol Chem 272:10004–10012
Blackburn AC, Tzeng HF, Anders MW, Board PG (2000) Discovery of a functional polymorphism in human glutathione transferase zeta by expressed sequence tag database analysis. Pharmacogenetics 10:49–57
Board PG, Baker RT, Chelvanayagam G, Jermiin LS (1997) Zeta, a novel class of glutathione transferases in a range of species from plants to humans. Biochem J 328:929–935
Bock KW, Fröhling W, Remmer H, Rexer B (1973) Effects of phenobarbital and 3-methylcholanthrene on substrate specificity of rat liver microsomal UDP-glucuronyltransferase. Biochim Biophys Acta 327:46–56
Coles BF, Morel F, Rauch C, Huber WW, Yang M, Teitel CH, Green B, Lang NP, Kadlubar FF (2001) Effect of polymorphism in the human glutathione S-transferase A1 promoter on hepatic GSTA1 and A2 expression. Pharmacogenetics 11:663–669
Dirr H, Reinemer P, Huber R (1994) X-ray crystal structures of cytosolic glutathione S-transferases. Implications for protein architecture, substrate recognition and catalytic function. Eur J Biochem 220:645–661
Eaton DL, Bammler TK (1999) Concise review of the glutathione S-transferases and their significance to toxicology. Toxicol Sci 49:156–164
Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapuric acid formation. J Biol Chem 249:7130–7139
Hayes JD, Pulford DJ (1995) The glutathione S-transferase supergene family: regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30:445–600
Hong SK, Anestis DK, Ball JG, Valentovic MA, Rankin GO (2002) In vitro nephrotoxicity induced by chloronitrobenzenes in renal cortical slices from Fischer 344 rats. Toxicol Lett 129:133–141
Igarashi T, Nanba E, Sagami F, Tsukidate K, Fukuda T, Horie T, Satoh T, Kitagawa H (1988) Dog liver glutathione S-transferase and its strong immunoreactivity with rat transferase-P(7-7). Biochem Pharmacol 37:4713–4718
Igarashi T, Kohara A, Shikata Y, Sagami F, Sonoda J, Horie T, Satoh T (1991) The unique feature of dog liver cytosolic glutathione S-transferases. An isozyme not retained on the affinity column has the highest activity toward 1,2-dichloro-4-nitrobenzene. J Biol Chem 266:21709–21717
Inskip A, Elexperu-Camiruaga J, Buxton N, Dias PS, MacIntosh J, Campbell D, Jones PW, Yengi L, Talbot JA, Strange RC, Fryer AA (1995) Identification of polymorphism at the glutathione S-transferase, GSTM3 locus: evidence for linkage with GSTM1*A. Biochem J 312:713–716
Ji X, von Rosenvinge EC, Johnson WW, Tomarev SI, Piatigorsky J, Armstrong RN, Gilliland GL (1995) Three-dimensional structure, catalytic properties, and evolution of a sigma class glutathione transferase from squid, a progenitor of the lens S-crystallins of cephalopods. Biochemistry 34:5317–5328
Knowles SR, Uetrecht J, Shear NH (2000) Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet 356:1587–1591
Landi S (2000) Mammalian class theta GST and differential susceptibility to carcinogens: a review. Mutat Res 463:247–283
Li Q, Minami M, Inagaki H (1998) Acute and subchronic immunotoxicity of p-chloronitrobenzene in mice. I. Effect of natural killer, cytotoxic T-lymphocyte activities and mitogen-stimulated lymphocyte proliferation. Toxicology 127:223–232
Li Q, Minami M, Hanaoka T, Yamamura Y (1999) Acute immunotoxicity of p-chloronitrobenzene in mice: II. Effect of p-chloronitrobenzene on the immunophenotype of murine splenocytes determined by flow cytometry. Toxicology 137:35–45
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265-275
Matsubara T, Otsubo S, Yoshihara E, Touchi A (1983) Biotransformation of coumarin derivatives (2). Oxidative metabolism of 7-alkoxycoumarin by microsomal enzymes and a simple assay procedure for 7-alkoxycoumarin O-dealkylase. Jpn J Pharmacol 33:41–56
Meyer DJ, Coles B, Pemble SE, Gilmore KS, Fraser GM, Ketterer B (1991) Theta, a new class of glutathione transferases purified from rat and man. Biochem J 274:409–414
Morrow CS, Cowan KH (1990) Glutathione S-transferases and drug resistance. Cancer Cells 2:15–22
Nair RS, Johannsen FR, Levinskas GJ, Terrill JB (1986a) Subchronic inhalation toxicity of p-nitroaniline and p-nitrochlorobenzene in rats. Fundam Appl Toxicol 6:618–627
Nair RS, Johannsen FR, Levinskas GJ, Terrill JB (1986b) Assessment of toxicity of o-nitrochlorobenzene in rats following a 4-week inhalation exposure. Fundam Appl Toxicol 7:609–614
Omura T, Sato R (1964) The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J Biol Chem 239:2370–2378
Pemble S, Schroeder KR, Spencer SR, Meyer DJ, Hallier E, Bolt HM, Ketterer B, Taylor JB (1994) Human glutathione S-transferase theta (GSTT1): cDNA cloning and the characterization of a genetic polymorphism. Biochem J 300:271–276
Pemble SE, Wardle AF, Taylor JB (1996) Glutathione S-transferase class Kappa: characterization by the cloning of rat mitochondrial GST and identification of a human homologue. Biochem J 319:749–754
Petersen KU (2002) From toxic precursors to safe drugs. Mechanisms and relevance of idiosyncratic drug reactions. Arzneimittelforschung 52:423–429
Pirmohamed M, Madden S, Park BK (1996) Idiosyncratic drug reactions. Metabolic bioactivation as a pathogenic mechanism. Clin Pharmacokinet 31:215–230
Prohaska JR, Ganther HE (1976) Glutathione peroxidase activity of glutathione S-transferases purified from rat liver. Biochem Biophys Res Commun 76:437–445
Pumford NR, Halmes NC (1997) Protein targets of xenobiotic reactive intermediates. Annu Rev Pharmacol Toxicol 37:91–117
Seidegard J, Vorachek WR, Pero RW, Pearson WR (1988) Hereditary differences in the expression of human glutathione transferase active on trans-stilbene oxide are due to a gene deletion. Proc Natl Acad Sci USA. 85:7293–7297
Shreve MR, Morrissey PG, O’Brien PJ (1979) Lipid and steroid hydroperoxides as substrates for the non-selenium-dependent glutathione peroxidase. Biochem J 177:761–763
Stücker I, Hirvonen A, de Waziers I, Cabelguenne A, Mitrunen K, Cenee S, Koum-Besson E, Hemon D, Beaune P, Loriot MA (2002) Genetic polymorphism of glutathione S-transferases as modulators of lung cancer susceptibility. Carcinogenesis 23:1475–1481
Toda G (1999) Liver injury induced by troglitazone. Endcrinol Diabetol 9:393–400
Travlos GS, Mahler J, Ragan, HA, Chou BJ, Bucher JR (1996) Thirteen-week inhalation toxicity of 2- and 4-chloronitrobenzene in F344/N rats and B6C3F1 mice. Fundam Appl Toxicol 30:75–92
van Poppel G, de Vogel N, van Balderen, PJ, Kok FJ (1992) Increased cytogenetic damage in smokers deficient in glutathione S-transferase isozyme mu. Carcinogenesis 13:303–305
Vlachodimitropoulos D, Norppa H, Autio K, Catalan J, Hirvonen A, Tasa G, Uuskula M, Demopoulos NA, Sorsa M (1997) GSTT1-dependent induction of centromere-negative and -positive micronuclei by 1,2:3,4-diepoxybutane in cultured human lymphocytes. Mutagenesis 12:397–403
Acknowledgements
We are particularly grateful to Dr. Koichi Hirano, Mr. Yasumitsu Nakatsugawa and Mr. Hitoshi Onishi for their advice and technical assistance. We also wish to express our thanks to Ms Caroline Bertorelli for proof-reading of this manuscript. We declare that this experiment complied with the current laws of Japan.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watanabe, T., Sugiura, T., Manabe, S. et al. Low glutathione S-transferase dogs. Arch Toxicol 78, 218–225 (2004). https://doi.org/10.1007/s00204-003-0536-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-003-0536-x