Abstract
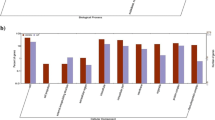
Listeria monocytogenes is a food-borne pathogen of humans and other animals. The striking ability to survive several stresses usually used for food preservation makes L. monocytogenes one of the biggest concerns to the food industry. This ubiquity can be partly explained by the ability of the organism to grow and persist at very low temperatures, a consequence of its ability to accumulate cryoprotective compound called osmolytes. A quantitative RT-PCR assay was used to measure mRNA transcript accumulation for the stress response genes opuCA and betL (encoding carnitine and betaine transporters, respectively) and the housekeeping gene 16S rRNA. Assays were conducted on mid-exponential phase L. monocytogenes cells exposed to conditions reflecting cold and freezing stress, conditions usually used to preserve foods. We showed that expression of the two cold-adapted genes encoded the transporters of the cryoprotectants carnitine and betaine in ATCC 19115 and the food-isolated L. monocytogenes S1 is induced after cold and freezing stress exposure. Furthermore, transcriptional analysis of the genes encoding opuCA and betL revealed that each transporter is induced to different degrees upon cold shock of L. monocytogenes ATCC 19115 and S1. Our results confirm an increase in carnitine uptake at low temperatures more than in betaine after cold-shocked temperature compared to the non-stress control treatment. It was concluded the use of carnitine and betaine as cryoprotectants is essential for rapid induction of the tested stress response under conditions typically encountered during food preservation.



Similar content being viewed by others

References
Abee T, Wouters JA (1999) Microbial stress response in minimal processing. Int J Food Microbiol 50:65–91
Barbuddhe SB, Chakraborty T (2009) Listeria as an enteroinvasive gastrointestinal pathogen. Curr Top Microbiol Immunol 337:173–195
Bayles DO, Annous BA, Wilkinson BJ (1996) Cold stress proteins induced in Listeria monocytogenes in response to temperature downshock and growth at low temperatures. Appl Environ Microbiol 62:1116–1119
Becker LA, Evans SN, Hutkins RW, Benson AK (2000) Role of σβ in adaptation of Listeria monocytogenes to growth at low temperature. J Bacteriol 182:7083–7087
Begley M, Gahan CG, Hill C (2002) Bile stress response in Listeria monocytogenes LO28: adaptation, cross-protection, and identification of genetic loci involved in bile resistance. Appl Environ Microbiol 68:6005–6012
Bergholz TM, Bowen B, Wiedmann M, Boor KJ (2012) Listeria monocytogenes shows temperature-dependent and—independent responses to salt stress, including responses that induce cross-protection against other stresses. Appl Environ Microbiol 78:2602–2612
Chan YC, Wiedmann M (2009) Physiology and genetics of Listeria monocytogenes survival and growth at cold temperatures. Crit Rev Food Sci Nutr 4:237–253
Chan YC, Raengpradub S, Boor KJ, Wiedmann M (2007) Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl Environ Microbiol 73:6484–6498
Fraser KR, Sue D, Wiedmann M, Boor K, O’Byrne CP (2003) Role of sigmaβ in regulating the compatible solute uptake systems of Listeria monocytogenes: osmotic induction of opuC is sigmaβ dependent. Appl Environ Microbiol 69:2015–2022
Gandhi M, Chikindas ML (2007) Listeria: a foodborne pathogen that knows how to survive. Int J Food Microbiol 113:1–15
Ko R, Smith LT (1999) Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl Environ Microbiol 65:4040–4048
Ko R, Smith LT, Smith GM (1994) Glycine betaine confers enhanced osmotolerance and cryotolerance on Listeria monocytogenes. J Bacteriol 176:426–431
Kramer MN, Coto D, Weidner JD (2005) The science of recalls. Meat Sci 71:158–163
Laksanalamai P, Burall LS, Datta AR (2010) Adaptation mechanisms of psychrotolerant bacterial pathogens. In: Horikoshi K, Antranikian G, Bull A, Robb F, Stetter K (eds) Extremophiles handbook, vol 2, 1st edn. Springer, Tokyo, pp 817–837
Larpent JP (1995) Les Listeria. Technique et documentation. Lavoisier, Paris, p 140
Leimeister-Wächter M, Domann E, Chakraborty T (1992) The expression of virulence genes in Listeria monocytogenes is thermoregulated. J Bacteriol 174:947–952
Liu S, Graham JE, Bigelow L, Morse PD 2nd, Wilkinson BJ (2002) Identification of Listeria monocytogenes genes expressed in response to growth at low temperature. Appl Environ Microbiol 68:1697–1705
Mendum ML, Smith LT (2002) Characterization of glycine betaine porter I from Listeria monocytogenes and its roles in salt and chill tolerance. Appl Environ Microbiol 68:813–819
Miladi H, Chaieb K, Bakhrouf A, Elmnasser N, Ammar E (2008) Freezing effects on survival of Listeria monocytogenes in artificially contaminated cold fresh-salmon. Ann Microbiol 58:471–476
Miladi H, Ammar E, Ben Slama R, Sakly N, Bakhrouf A (2013) Influence of freezing stress on morphological alteration and biofilm formation by Listeria monocytogenes: relationship with cell surface hydrophobicity and membrane fluidity. Arch Microbiol 195:705–715
Montville T, Matthews K (2008) Food microbiology: an introduction, 2nd edn. AMS press, Washington DC
Najjar MB, Chikindas M, Montville TJ (2007) Changes in Listeria monocytogenes membrane fluidity in response to temperature stress. Appl Environ Microbiol 73:6429–6435
O’Driscoll B (1997) Characterisation of the acid tolerance response in Listeria monocytogenes. Ph.D. thesis. National University of Ireland, Galway, Ireland
Olesen I, Vogensen FK, Jespersen L (2009) Gene transcription and virulence potential of Listeria monocytogenes strains after exposure to acidic and NaCl stress. Foodborne Pathog Dis 6:669–680
Posfay-Barbe KM, Wald ER (2009) Listeriosis. Semin Fetal Neonatal Med 14:228–233
Ramaswamy V, Cresence VM, Rejitha JS, Lekshmi MU, Dharsana KS, Prasad SP, Vijila HM (2007) Listeria-review of epidemiology and pathogenesis. J Microbiol Immunol Infect 40:4–13
Schmid B, Klumpp J, Raimann E, Loessner MJ, Stephan R, Tasara T (2009) Role of cold shock proteins in growth of Listeria monocytogenes under cold and osmotic stress conditions. Appl Environ Microbiol 75:1621–1627
Swaminathan B, Gerner-Smidt P (2007) The epidemiology of human listeriosis. Microbes Infect 9:1236–1243
Wemekamp-Kamphuis HH, Sleator RD, Wouters JA, Hill C, Abee T (2004) Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol 70:2912–2918
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Djamel Drider.
Rights and permissions
About this article
Cite this article
Miladi, H., Elabed, H., Ben Slama, R. et al. Molecular analysis of the role of osmolyte transporters opuCA and betL in Listeria monocytogenes after cold and freezing stress. Arch Microbiol 199, 259–265 (2017). https://doi.org/10.1007/s00203-016-1300-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1300-y