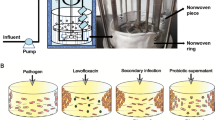
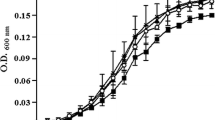
Abstract
Escherichia coli lives in the gastrointestinal tract and elsewhere, where it coexists within a mixed population. Indole production enables E. coli to grow with other gram-negative bacteria as indole inhibits N-acyl-homoserine lactone (AHL) quorum regulation. We investigated whether E. coli indole production enhanced competition with gram-positive Enterococcus faecalis, wherein quorum signaling is mediated by small peptides. During planktonic co-culture with E. faecalis, the fitness and population density of E. coli tnaA mutants (unable to produce indole) equaled or surpassed that of E. coli wt. During biofilm growth, the fitness of both populations of E. coli stabilized around 100 %, whereas the fitness of E. faecalis declined over time to 85–90 %, suggesting that biofilm and planktonic populations have different competition strategies. Media supplementation with indole removed the competitive advantage of E. coli tnaA in planktonic populations but enhanced it in biofilm populations. E. coli wt and tnaA showed similar growth in Luria–Bertani (LB) broth. However, E. coli growth was inhibited in the presence of filter-sterilized spent LB from E. faecalis, with inhibition being enhanced by indole. Similarly, there was also an inhibition of E. faecalis growth by proteinaceous components (likely bacteriocins) from spent culture media from both E. coli strains. We conclude that E. coli indole production is not a universal competition strategy, but rather works against gram-negative, AHL-producing bacteria.




Similar content being viewed by others
References
Baba T, Ara T, Hasegawa M, Takai Y, Okumura Y, Baba M, Datsenko KA, Tomita M, Wanner BL, Mori H (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol 2:2006.0008
Brown SA, Whiteley M (2007) A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans. J Bacteriol 189:6407–6414
Cascales E, Buchanan SK, Duche D, Kleanthous C, Lloubes R, Postle K, Riley M, Slatin S, Cavard D (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229
Cavera VL, Arthur TD, Kashtanov D, Chikindas ML (2015) Bacteriocins and their position in the next wave of conventional antibiotics. Int J Antimicrob Agents 46:494–501
Chandler JR, Heilmann S, Mittler JE, Greenberg EP (2012) Acyl-homoserine lactone-dependent eavesdropping promotes competition in a laboratory co-culture model. ISME J 6:2219–2228
Chu W, Zere TR, Weber MM, Wood TK, Whiteley M, Hidalgo-Romano B, Valenzuela E Jr, McLean RJC (2012) Indole production promotes Escherichia coli mixed culture growth with Pseudomonas aeruginosa by inhibiting quorum signaling. Appl Environ Microbiol 78:411–419
Conway T, Cohen PS (2015) Commensal and pathogenic Escherichia coli metabolism in the gut. Microbiol Spectr 3:MBP-0006-2014
Cook LC, Federle MJ (2014) Peptide pheromone signaling in Streptococcus and Enterococcus. FEMS Microbiol Rev 38:473–492
Culotti A, Packman AI (2014) Pseudomonas aeruginosa promotes Escherichia coli biofilm formation in nutrient-limited medium. PLoS One 9:e107186
Goh HF, Philip K (2015) Purification and characterization of bacteriocin produced by Weissella confusa A3 of dairy origin. PLoS One 10:e0140434
Golberg K, Pavlov V, Marks RS, Kushmaro A (2013) Coral-associated bacteria, quorum sensing disrupters, and the regulation of biofouling. Biofouling 29:669–682
Hidalgo-Romano B, Gollihar J, Brown SA, Whiteley M, Valenzuela E Jr, Kaplan HB, Wood TK, McLean RJC (2014) Indole inhibition of AHL-mediated quorum signaling is widespread in gram-negative bacteria. Microbiology 160:2464–2473
Kawamura-Sato K, Shibayama K, Horii T, Iimuma Y, Arakawa Y, Ohta M (1999) Role of multiple efflux pumps in Escherichia coli in indole expulsion. FEMS Microbiol Lett 179:345–352
Khare A, Tavazoie S (2015) Multifactorial competition and resistance in a two-species bacterial system. PLoS Genet 11:e1005715
Kirkup BC, Riley MA (2004) Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature 428:412–414
Kommineni S, Bretl DJ, Lam V, Chakraborty R, Hayward M, Simpson P, Cao Y, Bousounis P, Kristich CJ, Salzman NH (2015) Bacteriocin production augments niche competition by enterococci in the mammalian gastrointestinal tract. Nature 526:719–722
Korgaonkar A, Trivedi U, Rumbaugh K, Whiteley M (2012) Community surveillance enhances Pseudomonas aeruginosa virulence during polymicrobial infection. Proc Natl Acad Sci USA 110:1059–1064
Lee JH, Lee J (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol Rev 34:426–444
Lin YC, Lin WP, Huang JY, Lee SY (2012) Polymicrobial peritonitis following colonoscopic polypectomy in a peritoneal dialysis patient. Intern Med 51:1841–1843
Macleod SM, Stickler DJ (2007) Species interactions in mixed-community crystalline biofilms on urinary catheters. J Med Microbiol 56:1549–1557
McLellan SL, Huse SM, Mueller-Spitz SR, Andreishcheva EN, Sogin ML (2010) Diversity and population structure of sewage-derived microorganisms in wastewater treatment plant influent. Environ Microbiol 12:378–392
Palmer KL, Mashburn LM, Singh PK, Whiteley M (2005) Cystic fibrosis sputum supports growth and cues key aspects of Pseudomonas aeruginosa physiology. J Bacteriol 187:5267–5277
Palmer KL, Godfrey P, Griggs A, Kos VN, Zucker J, Desjardins C, Cerqueira G, Gevers D, Walker S, Wortman J, Feldgarden M, Haas B, Birren B, Gilmore MS (2012) Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. mBio 3:e00318-11
Smibert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhardt P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington DC, pp 607–654
Tagg JR, McGiven AR (1971) Assay system for bacteriocins. Appl Microbiol 21:943
The Human Microbiome Project Consortium (2012) A framework for human microbiome research. Nature 486:215–221
Ushijima T, Seto A (1991) Selected faecal bacteria and nutrients essential for antagonism of Salmonella typhimurium in anaerobic continuous flow cultures. J Med Microbiol 35:111–117
Vijayakumar PP, Muriana PM (2015) A microplate growth inhibition assay for screening bacteriocins against Listeria monocytogenes to differentiate their mode-of-action. Biomolecules 5:1178–1194
Wanjugi P, Harwood VJ (2013) The influence of predation and competition on the survival of commensal and pathogenic fecal bacteria in aquatic habitats. Environ Microbiol 15:517–526
Weber MM, French CL, Barnes MB, Siegele DA, McLean RJC (2010) A previously uncharacterized gene, yjfO (bsmA) influences Escherichia coli biofilm formation and stress response. Microbiology 156:139–147
Acknowledgments
This work was funded by a grant from the Research Enhancement Program at Texas State University to RJCM. We thank the reviewers for helpful suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Djamel Drider.
Rights and permissions
About this article
Cite this article
Pringle, S.L., Palmer, K.L. & McLean, R.J.C. Indole production provides limited benefit to Escherichia coli during co-culture with Enterococcus faecalis . Arch Microbiol 199, 145–153 (2017). https://doi.org/10.1007/s00203-016-1289-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1289-2