Abstract
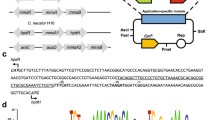
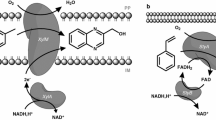
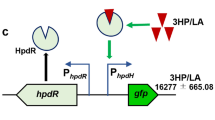
This work reports the preparation of two recombinant strains each containing two enzymatic activities mutually expressed through regulated systems for production of functionalized epoxides in one-pot reactions. One strain was Pseudomonas putida PaW340, containing the gene coding for styrene monooxygenase (SMO) from Pseudomonas fluorescens ST under the auto-inducing Ptou promoter and the TouR regulator of Pseudomonas sp. OX1 and the gene coding for naphthalene dihydrodiol dehydrogenase (NDDH) from P. fluorescens N3 under the Ptac promoter inducible by IPTG. The second strain was Escherichia coli JM109, in which the expression of SMO was under the control of the Pnah promoter and the NahR regulator of P. fluorescens N3 inducible by salicylate, while the gene expressing NDDH was under the control of the Plac promoter inducible by IPTG. SMO and NDDH activities were tested in bioconversion experiments using cinnamyl alcohol as reference substrate. The application that we selected is one example of the sequential use of the two enzymatic activities which require a temporal control of the expression of both genes.









Similar content being viewed by others
References
Alcalde M, Ferrer M, Plou FJ, Ballesteros A (2006) Environmental biocatalysis: from remediation with enzymes to novel green processes. Trends Biotechnol 24:281–287
Arenghi FLD, Berlanda D, Galli E, Sello G, Barbieri P (2001) Organization and regulation of meta cleavage pathway gene for toluene and o-xylene derivative degradation in Pseudomonas stutzeri OX1. Appl Environ Microbiol 67:3304–3308
Bernasconi S, Orsini F, Sello G, Di Gennaro P (2004) Bacterial monooxygenase mediated preparation of chiral oxiranes: study of the effects of substituent nature and position. Tetrahedron Asymmetry 15:1603–1606
Bestetti G, Di Gennaro P, Galli E, Bernasconi S, Orsini F, Sello G (2003) Development of bioconversion processes by biocatalysts based on Pseudomonas oxygenases to produce oxygenated compounds. Recent Res Dev Appl Microbiol Biotechnol 1:197–217
Chartrain M, Salmon PM, Robinson DK, Buckland BC (2000) Metabolic engineering and directed evolution for production of pharmaceuticals. Curr Opin Biotechnol 11:209–214
De Lorenzo V, Eltis L, Kessler B, Timmis KN (1993) Analysis of Pseudomonas gene products using lacIq/Ptrp-lac plasmids and trasposons that confer conditional phenotypes. Gene 23:17–24
De Wildeman SMA, Sonke T, Schoemaker HE, May O (2007) Biocatalytic reductions: from lab curiosity to first choice. Chem Res 40:1260–1266
Di Gennaro P, Galli E, Albini G, Pelizzoni F, Sello G, Bestetti G (1997) Production of substituted naphthalene dihydrodiols by engineered Escherichia coli containing the cloned naphthalene 1,2-dioxygenase gene from Pseudomonas fluorescens N3. Res Microbiol 148:355–364
Di Gennaro P, Colmegna A, Galli E, Sello G, Pelizzoni F, Bestetti G (1999) A new biocatalyst for production of optically pure aryl epoxides by styrene monooxygenase from Pseudomonas fluorescens ST. Appl Environ Microbiol 65:2794–2797
Di Gennaro P, Galli E, Orsini F, Pelizzoni F, Sello G, Bestetti G (2000) Development of biocatalysts carrying naphthalene dioxygenase and dihydrodiol dehydrogenase genes inducible in aerobic and anaerobic conditions. Res Microbiol 151(5):383–391
Di Gennaro P, Ferrara S, Bestetti G, Sello G, Solera D, Galli E, Renzi F, Bertoni G (2008) Novel auto-inducing expression systems for the development of whole-cell biocatalysts. Appl Microbiol Biotechnol 79:617–625
Faber K, Patel R (2000) Chemical biotechnology. A happy marriage between chemistry and biotechnology: asymmetric synthesis via green chemistry. Curr Opin Biotechnol 11:517–551
Felfer U, Gorlup M, Koegl MF, Wagner U, Larissegger-Schnell B, Faber K, Kroutil W (2005) The substrate spectrum of mandelate racemase: minimum structural requirements for substrates and substrate model. Adv Synth Catal 347:951–961
Franklin FC, Williams PA (1980) Construction of a partial diploid for the degradative pathway encoded by the TOL plasmid from Pseudomonas putida mt-2: evidence for the positive nature of the regulation by the xylR gene. Mol Gen Genet 177:321–328
Hashimoto S, Ozaki A (1999) Whole microbial cell processes for manufacturing amino acids, vitamins or ribonucleotides. Curr Opin Biotechnol 10:604–608
Hibbert EG, Baganz F, Hailes HC, Ward JM, Lye GJ, Woodley JM, Dalby PA (2005) Directed evolution of biocatalytic processes. Biomol Eng 22:11–19
Julsing MK, Kuhn D, Schmid A, Bűhler B (2012) Resting cells of recombinant E. coli show high epoxidation yields on energy source and high sensitivity to product inhibition. Biotechnol Bioeng 109(5):1109–1119
Koszelewski D, Lavandera I, Clay D, Rozzel I, Kroutil W (2008) Asymmetric synthesis of optically pure pharmacologically relevant amines employing omega-transaminases. Adv Synth Catal 350:2761–2766
Lopez-Gallego F, Schmidt-Dannert C (2009) Multi-enzymatic synthesis. Curr Opin Chem Biol 14:174–183
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Monti D, Ferrandi EE, Zanellato I, Hua L, Polentini F, Carrea G, Riva S (2009) One-pot multienzymatic synthesis of 12-ketoursodeoxycholic acid: subtle cofactor specificities rule the reaction equilibria of five biocatalysts working in a row. Adv Synth Catal 351:1303–1311
Panke S, Wubbolts MG, Witholt B (2000) Production of enantiopure styrene oxide by recombinant Escherichia coli synthesizing a two-component styrene monooxygenase. Biotechnol Bioeng 69:91–100
Park JB, Buhler B, Panke S, Witholt B, Schmid A (2007) Carbon metabolism and product inibition determine the epoxydation efficiency of solvent tolerant Pseudomonas sp. strain VLB129∆C. Biotechnol Bioeng 98(6):1219–1229
Plucinska K, Kasprzykowski F, Kozian E (1997) Synthesis of enantiomerically pure forms of trans-3-phenylglycidic acid. Tetrahedron Lett 38(5):861–864
Sambrook J, Russel D (2000) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Schmid A, Dordick JS, Hauer B, Kiener A, Wubbolts MG, Witholt B (2001) Industrial biocatalysis today and tomorrow. Nature 409:258–268
Seisser B, Lavandera I, Faber K, Lutje Spelberg JH, Kroutil W (2007) Stereo-complementary two-step cascades using a two-enzyme system leading to enantiopure epoxides. Adv Synth Catal 349:1399–1404
Sello G, Bernasconi S, Orsini F, Mattavelli P, Di Gennaro P, Bestetti G (2008) Biocatalyst expressing cis-naphthalene dihydrodiol dehydrogenase from Pseudomonas fluorescens N3 catalyzes alcohol and 1,2-diol dehydrogenase reactions. J Mol Cat B Enzym 52:67–73
Sello G, Bernasconi S, Orsini F, Di Gennaro P (2009) Multienzymatic preparation of (−)-[3-(oxiran-2-yl) phenyl] methanol and (−)-3-(oxyran-2-yl) benzoic acid. Tetrahedron Asymmetry 20:563–565
Voss CV, Gruber CC, Faber K, Knaus T, Macheroux P, Kroutil W (2008) Orchestration of concurrent oxidation and reduction cycles for stereoinversion and deracemization of sec-alcohols. J Am Chem Soc 130:13969–13972
Wong CH, Whitesides GM (1982) Enzyme-catalyzed organic synthesis: NAD(P)H cofactor regeneration using ethanol/alcohol dehydrogenase/aldehyde dehydrogenase and methano/alcohol dehydrogenase/aldehyde dehydrogenase/formate dehydrogenase. J Org Chem 47:2816–2818
Yanish-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103–119
Acknowledgments
This work was supported by MIUR-PRIN 2007-2009 (Grant 20077MY8M9 004) and by MIUR-FAR 2011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Eriko Takano.
Rights and permissions
About this article
Cite this article
Di Gennaro, P., Kazandjian, L.V., Mezzetti, F. et al. Regulated expression systems for the development of whole-cell biocatalysts expressing oxidative enzymes in a sequential manner. Arch Microbiol 195, 269–278 (2013). https://doi.org/10.1007/s00203-013-0875-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-013-0875-9