Abstract
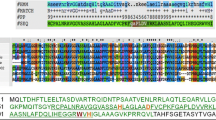
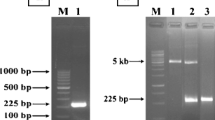
BhlA, a putative holin-like protein of Bacillus licheniformis AnBa9 expressed in Escherichia coli BL21(DE3) showed antibacterial activity against several gram-positive bacteria including methicillin-resistant Staphylococcus aureus (MRSA) and Micrococcus luteus. Deletion analysis of bhlA suggests that a hydrophobic transmembrane domain, BhlATM is essential for antibacterial activity. Though the minimum inhibitory concentration (MIC) of BhlA was sevenfold lower than BhlATM, transmembrane domain deleted construct (BhlA∆TM) had no antibacterial activity. The expression of BhlA in E. coli was found to be toxic to cells. Therefore, the bhlA was cloned in yeast surface display vector pYD1 and expressed in Saccharomyces cerevisiae. The surface displayed yeast showed inhibition of several gram-positive bacteria. This recombinant yeast expressing BhlA may be used as biodrug for efficient control of multiple drug-resistant bacterial infections.




Similar content being viewed by others
References
Anthony T, Rajesh T, Kayalvizhi N, Gunasekaran P (2009) Influence of medium components and fermentation conditions on the production of bacteriocin(s) by Bacillus licheniformis AnBa9. Bioresour Technol 100:872–877
Bläsi U, Nam K, Hartz D, Gold L, Young R (1989) Dual translational start sites control function of the lambda S gene. EMBO J 8:3501–3510
Bläsi U, Fraisl P, Chang CY, Zhang N, Young R (1999) The C-terminal sequence of the lambda holin constitutes a cytoplasmic regulatory domain. J Bacteriol 181:2922–2929
Bonovich MT, Young R (1991) Dual start motif in two lambdoid S genes unrelated to λS. J Bacteriol 173:2897–2905
Gietz RD, Woods RA (2001) Genetic transformation of yeast. Bio-Techniques 30:816–820, 822–826, 828
Kruszewska D, Sahl HG, Bierbaum G, Pag U, Hynes SO, Ljungh A (2004) Mersacidin eradicates methicillin-resistant Staphylococcus aureus (MRSA) in a mouse rhinitis model. J Antimicrob Chemother 54:648–653
Murdoch DR, Mirrett S, Harrell LJ, Donabedian SM, Zervos MJ, Reller LB (2003) Comparison of microscan broth microdilution, synergy quad plate agar dilution, and disk diffusion screening methods for detection of high-level aminoglycoside resistance in Enterococcus Species. J Clin Microbiol 41:2703–2705
Natasa V, Isabella M, Bläsi U, Siegfried S, Martin JM (2003) Functional regulation of the Listeria monocytogenes bacteriophage A118 holin by an intragenic inhibitor lacking the first transmembrane domain. Mol Microbiol 48:173–186
Nikaido H, Vaara M (1985) Molecular basis of bacterial outer membrane permeability. Microbiol Mol Biol Rev 49:1–32
Takáč M, Witte A, Bläsi U (2005) Functional analysis of the lysis genes of Staphylococcus aureus phage P68 in Escherichia coli. Microbiol 151:2331–2342
Tran TA, Struck DK, Young R (2005) Periplasmic domains define holin-antiholin interactions in T4 lysis inhibition. J Bacteriol 187:6631–6640
Young R, Bläsi U (1995) Holins: form and function in bacteriophage lysis. FEMS Microbiol Rev 17:191–205
Žiedaitė G, Daugelavičius R, Bamford JKH, Bamford DH (2004) The holin protein of bacteriophage PRD1 forms a pore for small-molecule and endolysin translocation. J Bacteriol 187:5397–5405
Acknowledgments
This work was supported by a grant from the Department of Science and Technology, India under OYS (SR/FT/L-98/2004) to TA. Support facility from Centre for Excellence in Genomic Sciences, Networking Resource Centre in Biological Sciences, Madurai Kamaraj University is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jorge Membrillo-Hernandez.
G. S. Chellappa and T. Rajesh have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Anthony, T., Chellappa, G.S., Rajesh, T. et al. Functional analysis of a putative holin-like peptide-coding gene in the genome of Bacillus licheniformis AnBa9. Arch Microbiol 192, 51–56 (2010). https://doi.org/10.1007/s00203-009-0530-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-009-0530-7