Abstract
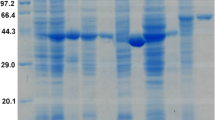
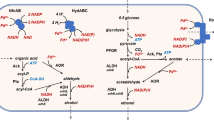
Thermotoga hypogea is an extremely thermophilic anaerobic bacterium capable of growing at 90°C. It uses carbohydrates and peptides as carbon and energy sources to produce acetate, CO2, H2, l-alanine and ethanol as end products. Alcohol dehydrogenase activity was found to be present in the soluble fraction of T. hypogea. The alcohol dehydrogenase was purified to homogeneity, which appeared to be a homodimer with a subunit molecular mass of 40 ± 1 kDa revealed by SDS-PAGE analyses. A fully active enzyme contained iron of 1.02 ± 0.06 g-atoms/subunit. It was oxygen sensitive; however, loss of enzyme activity by exposure to oxygen could be recovered by incubation with dithiothreitol and Fe2+. The enzyme was thermostable with a half-life of about 10 h at 70°C, and its catalytic activity increased along with the rise of temperature up to 95°C. Optimal pH values for production and oxidation of alcohol were 8.0 and 11.0, respectively. The enzyme had a broad specificity to use primary alcohols and aldehydes as substrates. Apparent Km values for ethanol and 1-butanol were much higher than that of acetaldehyde and butyraldehyde. It was concluded that the physiological role of this enzyme is likely to catalyze the reduction of aldehydes to alcohols.




Similar content being viewed by others
Abbreviations
- ADH:
-
Alcohol dehydrogenase
- CAPS:
-
3-(Cyclohexylamino)-1-propanesulfonic acid
- EDTA:
-
Ethylenediaminetetraacetic acid
- HEPES:
-
4-(2-Hydroxyethyl)-1-piperazineethanesulfonic acid
- ITV-ICP-AES:
-
In-torch vaporization-inductively coupled plasma-atomic emission spectrometry
- PIPES:
-
1,4-Piperazine-bis-(ethanesulfonic acid)
References
Antoine E, Rolland JL, Raffin JP, Dietrich J (1999) Cloning and over-expression in Escherichia coli of the gene encoding NADPH group III alcohol dehydrogenase from Thermococcus hydrothermomalis. Eur J Biochem 264:880–889
Badiei HR, Smith AT, Karanassios V (2002) Rhenium-cup, in-torch vaporization (ITV) sample introduction for axially viewed ICP-AES and its application to the analysis of a microscopic, ng-weight solid sample. J Anal At Spectrom 17:1030–1036
Balk M, Weijma J, Stams AJM (2002) Thermotoga lettingae sp. nov., a novel thermophilic, methanol-degrading bacterium isolated from a thermophilic anaerobic reactor. Int J Syst Evol Microbiol 52:1361–1368
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brinen LS, Canaves JM, Dai X, Deacon AM, Elsliger MA, Eshaghi S, Floyd R, Godzik A, Grittini C, Grzechnik SK, Guba C, Jaroszewski L, Karlak C, Klock HE, Koesema E, Kovarik JS, Kreusch A, Kuhn P, Lesley SA, McMullan D, McPhillips TM, Miller MA, Miller MD, Morse A, Moy K, Ouyang J, Robb A, Rodrigues K, Selby TL, Spraggon G, Stevens RC, van den Bedem H, Velasquez J, Vincent J, Wang X, West B, Wolf G, Taylor SS, Hodgson KO, Wooley J, Wilson IA (2002) Crystal structure of a zinc-containing glycerol dehydrogenase (TM0423) from Thermotoga maritima at 1.5 Å resolution. Proteins 50:371–374
Copeland A, Lucas S, Lapidus A, Barry K, Glavina del Rio T, Dalin E, Tice H, Bruce D, Pitluck S, Richardson P (2006a) Sequencing of the draft genome and assembly of Fervidobacterium nodosum Rt17-B1. US DOE Joint Genome Institute (JGI-PGF). http://www.genome.jgi-psf.org/draft_microbes/ferno/ferno.home.html accessed on 29 December, 2006
Copeland A, Lucas S, Lapidus A, Barry K, Glavina del Rio T, Dalin E, Tice H, Bruce D, Pitluck S, Richardson P (2006b) Sequencing of the draft genome and assembly of Thermotoga petrophila RKU-1. US DOE Joint Genome Institute (JGI-PGF). http://www.genome.jgi-psf.org/draft_microbes/thepr/thepr.home.html accessed on 29 December, 2006
Copeland A, Lucas S, Lapidus A, Barry K, Glavina del Rio T, Dalin E, Tice H, Bruce D, Pitluck S, Richardson P (2006c) Sequencing of the draft genome and assembly of Thermoanaerobacter ethanolicus X514. US DOE Joint Genome Institute (JGI-PGF). http://www.genome.jgi-psf.org/draft_microbes/theex/theex.home.html accessed on 29 December, 2006
Deutscher MP (1990) Guide to protein purification. Methods Enzymol 182:588–604
Esposito L, Sica F, Raia CA, Giordano A, Rossi M, Mazzarella L, Zagari A (2002) Crystal structure of the alcohol dehydrogenase from the hyperthermophilic archaeon Sulfolobus solfataricus at 1.85 Å resolution. J Mol Biol 318:463–477
Fardeau ML, Ollivier B, Patel BKC, Magot M, Thomas P, Rimbault A, Rocchiccioli F, Garcia JL (1997) Thermotoga hypogea sp. nov., a xylanolytic, thermophilic bacterium from an oil-producing well. Int J Syst Bacteriol 47:1013–1019
Guagliardi A, Martino M, Iaccarino I, Rosa MD, Rossi M, Bartolucci S (1996) Purification and characterization of the alcohol dehydrogenase from a novel strain of Bacillus stearothermophilus growing at 70°C. Int J Biochem Cell Biol 28:239–246
Guy JE, Isupov MN, Littlechild JA (2003) The structure of an alcohol dehydrogenase from the hyperthermophilic archaeon Aeropyrum pernix. J Mol Biol 331:1041–1051
Hirakawa H, Kamiya N, Kawarabayashi Y, Nagamune T (2004) Properties of an alcohol dehydrogenase from the hyperthermophilic archaeon Aeropyrum pernix K1. J Biosci Bioeng 97:202–206
Ingram LO, Aldrich HC, Borges ACC, Causey TB, Martinez A, Morales F, Saleh A, Underwood SA, Yomano LP, York SW, Zaldivar J, Zhou S (1999) Enteric bacterial catalysts for fuel ethanol production. Biotechnol Prog 15:855–866
Jochimsen B, Peinemann-Simon S, Völker H, Stüben D, Botz R, Stoffers P, Dando PR, Thomm M (1997) Stetteria hydrogenophila, gen. nov. and sp. nov., a novel mixotrophic sulfur-dependent crenarchaeote isolated from Milos, Greece. Extremophiles 1:67–73
Kelly RM, Adams MWW (1994) Metabolism in hyperthermophilic microorganisms. Antonie Van Leeuwenhoek 66:247–270
Kengen SWM, de Bok FAM, van Loo ND, Dijkema C, Stams AJM, de Vos WM (1994) Evidence for the operation of a novel Embden–Meyerhof pathway that involves ADP-dependent kinases during sugar fermentation by Pyrococcus furiosus. J Biol Chem 269:17537–17541
Kort R, Liebl W, Labedan B, Forterre P, Eggen RIL, de Vos WM (1997) Glutamate dehydrogenase from hyperthermophilic bacterium Thermotoga maritima: molecular characterization and phylogenetic implications. Extremophiles 1:52–60
Laemmli UK (1970) Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature 227:680–685
Littlechild JA, Guy JE, Isupov MN (2004) Hyperthermophilic dehydrogenase enzymes. Biochem Soc Trans 32:255–258
Ma K, Adams MWW (1999) An unusual oxygen-sensitive, iron- and zinc-containing alcohol dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. J Bacteriol 181:1163–1170
Ma K, Robb FT, Adams MWW (1994) Purification and characterization of NADP-specific alcohol dehydrogenase and glutamate dehydrogenase from the hyperthermophilic archaeon Thermococcus litoralis. Appl Environ Microbiol 60:562–568
Ma K, Loessner H, Heider J, Johnson MK, Adams MWW (1995) Effects of elemental sulfur on the metabolism of the deep-sea hyperthermophilic archaeon Thermococcus strain ES-1: characterization of a sulfur-regulated, non-heme iron alcohol dehydrogenase. J Bacteriol 177:4748–4756
Ma K, Hutchins A, Sung SJS, Adams MWW (1997) Pyruvate ferrodoxin oxidoreductase from the hyperthermophilic archaeon, Pyrococcus furiosus, functions as a CoA-dependent pyruvate decarboxylase. Proc Natl Acad Sci 94:9608–9613
Nelson KE, Clayton R, Gill SR, Gwinn ML, Dodson RJ, Haft DH, Hickey EK, Peterson J, Nelson WC, Ketchum KA, McDonald L, Utterback TR, Malek JA, Linher KD, Garrett MM, Stewart AM, Cotton MD, Pratt MS, Phillips CA, Richardson D, Heidelberg J, Sutton GG, Fleischmann RD, White O, Salzberg SL, Smith HO, Venter JC, Fraser CM (1999) Evidence for lateral gene transfer between archaea and bacteria from genome sequence of Thermotoga maritima. Nature 399:323–329
van der Oost J, Voorhorst WGB, Kengen SWM, Geerling ACM, Wittenhorst V, Gueguen Y, de Vos WM (2001) Genetic and biochemical characterization of a short-chain alcohol dehydrogenase from the hyperthermophilic archaeon Pyrococcus furiosus. Eur J Biochem 268:3062–3068
Pan N, Imlay JA (2001) How does oxygen inhibit central metabolism in the obligate anaerobe Bacteroides thetaiotaomicron? Mol Microbiol 39:1562–1571
Patel BKC, Morgan HW, Daniel RM (1985) Fervidobacterium nodosum gen. nov. and spec. nov. a new chemoorganotrophic, caldoactive, anaerobic bacterium. Arch Microbiol 141:63–69
Radianingtyas H, Wright PC (2003) Alcohol dehydrogenases from thermophilic and hyperthermophilic archaea and bacteria. FEMS Microbiol Rev 794:1–24
Reid MF, Fewson CA (1994) Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol 20:13–56
Rella R, Raia CA, Pensa M, Pisani FM, Gambacorta A, De Rosa M, Rossi M (1987) A novel archaebacterial NAD+-dependent alcohol dehydrogenase: purification and properties. Eur J Biochem 167:475–479
Robb FT, Maeder DL (1998) Novel evolutionary histories and adaptive features of proteins from hyperthermophiles. Curr Opin Biotech 9:288–291
Robb FT, Park JB, Adams MWW (1992) Characterization of an extremely thermostable glutamate dehydrogenase: a key enzyme in the primary metabolism of the hyperthermophilic archaebacterium, Pyrococcus furiosus. Biochim Biophys Acta 1120:267–272
Robb FT, Maeder DL, DiRuggiero J, Borges KM, Tolliday N (2001) Glutamate dehydrogenases from hyperthermophiles. In: Adams MWW, Kelly RM (eds) Methods in enzymology, vol 331. Academic, New York, pp 26–41
Sakuraba H, Goda S, Ohshima T (2004) Unique sugar metabolism and novel enzymes of hyperthermophilic archaea. Chem Rec 3:281–287
Schönheit P, Schäfer T (1995) Metabolism of hyperthermophiles. World J Microbiol Biotechnol 11:26–57
Schwarzenbacher R, von Delft F, Canaves JM, Brinen LS, Dai X, Deacon AM, Elsliger MA, Eshaghi S, Floyd R, Godzik A, Grittini C, Grzechnik SK, Guba C, Jaroszewski L, Karlak C, Klock HE, Koesema E, Kovarik JE, Kreusch A, Kuhn P, Lesley SA, McMullan D, McPhillips TM, Miller MA, Miller MD, Morse A, Moy K, Ouyang J, Page R, Robb A, Rodrigues K, Selby TL, Spraggon G, Stevens RC, van den Bedem H, Velasquez J, Vincent J, Wang X, West B, Wolf G, Hodgson KO, Wooley J, Wilson IA (2004) Crystal structure of an iron-containing 1,3-propanediol dehydrogenase (TM0920) from Thermotoga maritima at 1.3 Å resolution. Proteins 54:174–177
Scopes PK (1983) An iron-activated alcohol dehydrogenase. FEBS Lett 156:303–306
Selig M, Xavier KB, Santos H, Schönheit P (1997) Comparative analysis of Embden–Meyerhof and Entner–Doudoroff glycolytic pathways in hyperthermophilic archaea and the bacterium Thermotoga. Arch Microbiol 167:217–232
Sheehan JJ (1994) Bioconversion for production of renewable transportation fuels in the United States: a strategic perspective. In: Himmel ME, Baker JO, Overend RP (eds) Enzymatic conversion of biomass for fuels production, ACS symposium series 566. American Chemical Society, Washington DC, pp 1–53
Siebers B, Schönheit P (2005) Unusual pathways and enzymes of central carbohydrate metabolism in archaea. Curr Opin Microbiol 8:695–705
Stetter KO (1989) Extremely thermophilic chemolithoautotrophic archaebacteria. In: Schlegel HG, Bowien B (eds) Autotrophic bacteria, Science Tech Publishers/Springer, Madison/Berlin, pp 167–176
Stetter KO (1996) Hyperthermophilic prokaryotes. FEMS Microbiol Rev 18:149–158
Ueda K, Yamashita A, Ishikawa J, Shimada M, Watsuji TO, Morimura K, Ikeda H, Hattori M, Beppu T (2004) Genome sequence of Symbiobacterium thermophilum, an uncultivable bacterium that depends on microbial commensalisms. Nucleic Acids Res 32:4937–4944
de Vos WM, Kengen SWM, Voorhorst WGB, van der Oost J (1998) Sugar utilization and its control in hyperthermophile. Extremophiles 2:201–205
Verhees CH, Kengen SW, Tuininga JE, Schut GJ, Adams MW, de Vos WM, van der Oost J (2003) The unique features of glycolytic pathways in archaea. Biochem J 375:231–246
Wiegel J, Ljungdahl LG (1981) Thermoanaerobacter ethanolicus gen. nov., spec. nov., a new, extremely thermophilic, anaerobic bacterium. Arch Microbiol 128:343–348
Yang X, Ma K (2005) Purification and characterization of an NADH oxidase from extremely thermophilic anaerobic bacterium Thermotoga hypogea. Arch Microbiol 183:331–337
Ziegenhorn J, Senn M, Bücher T (1976) Molar absorptivities of β-NADH and β-NADPH. Clin Chem 22(2):151–160
Acknowledgments
This work was supported by research grants from Ontario Ministry of Agriculture and Food, Rural Affairs, Natural Sciences and Engineering Research Council (Canada) and Canada Foundation for Innovation, and funds from the University of Waterloo to KM. We thank Feng Zhang for helping grow T. hypogea and Xianqin Yang for measuring glutamate dehydrogenase activities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ying, X., Wang, Y., Badiei, H.R. et al. Purification and characterization of an iron-containing alcohol dehydrogenase in extremely thermophilic bacterium Thermotoga hypogea . Arch Microbiol 187, 499–510 (2007). https://doi.org/10.1007/s00203-007-0217-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-007-0217-x