Abstract
Summary
To predict the burden of incident osteoporosis attributable fractures (OAF) in Germany, an economic simulation model was built. The burden of OAF will sharply increase until 2050. Future demand for hospital and long-term care can be expected to substantially rise and should be considered in future healthcare planning.
Introduction
The aim of this study was to develop an innovative simulation model to predict the burden of incident OAF occurring in the German population, aged >50, in the time period of 2010 to 2050.
Methods
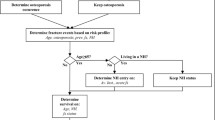
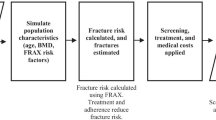
A Markov state transition model based on five fracture states was developed to estimate costs and loss of quality adjusted life years (QALYs). Demographic change was modelled using individual generation life tables. Direct (inpatient, outpatient, long-term care) and indirect fracture costs attributable to osteoporosis were estimated by comparing Markov cohorts with and without osteoporosis.
Results
The number of OAF will rise from 115,248 in 2010 to 273,794 in 2050, cumulating to approximately 8.1 million fractures (78 % women, 22 % men) during the period between 2010 and 2050. Total undiscounted incident OAF costs will increase from around 1.0 billion Euros in 2010 to 6.1 billion Euros in 2050. Discounted (3 %) cumulated costs from 2010 to 2050 will amount to 88.5 billion Euros (168.5 undiscounted), with 76 % being direct and 24 % indirect costs. The discounted (undiscounted) cumulated loss of QALYs will amount to 2.5 (4.9) million.
Conclusions
We found that incident OAF costs will sharply increase until the year 2050. As a consequence, a growing demand for long-term care as well as hospital care can be expected and should be considered in future healthcare planning. To support decision makers in managing the future burden of OAF, our model allows to economically evaluate population- and risk group-based interventions for fracture prevention in Germany.


Similar content being viewed by others
Notes
Referring to cost ranges (± percent from mean costs) univariate distribution were used.
References
Statistisches Bundesamt (2009) Bevölkerung Deutschland bis 2060. 12. koordinierte Bevölkerungsvorausberechnung [Population in Germany up to the year 2060. Results of the 12th coordinated projection of population]. Statistisches Bundesamt, Wiesbaden
Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A (2000) Risk of hip fracture according to the World Health Organization criteria for osteopenia and osteoporosis. Bone 27:585–590
World Health Organisation (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. World Health Organ Tech Rep Ser 843:1–129
Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, Eisman JA, Fujiwara S, Kroger H, Mellstrom D, Meunier PJ, Melton LJ 3rd, O’Neill T, Pols H, Reeve J, Silman A, Tenenhouse A (2005) Predictive value of BMD for hip and other fractures. J Bone Miner Res 20:1185–1194
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312:1254–1259
Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, Nevitt MC, Cummings SR (2003) BMD at multiple sites and risk of fracture of multiple types: long-term results from the Study of Osteoporotic Fractures. J Bone Miner Res 18:1947–1954
Osnes EK, Lofthus CM, Meyer HE, Falch JA, Nordsletten L, Cappelen I, Kristiansen IS (2004) Consequences of hip fracture on activities of daily life and residential needs. Osteoporos Int 15:567–574
Hiligsmann M, Ethgen O, Richy F, Reginster JY (2008) Utility values associated with osteoporotic fracture: a systematic review of the literature. Calcif Tissue Int 82:288–292
Morin S, Lix LM, Azimaee M, Metge C, Caetano P, Leslie WD (2011) Mortality rates after incident non-traumatic fractures in older men and women. Osteoporos Int 22:2439–2448
Budhia S, Mikyas Y, Tang M, Badamgarav E (2012) Osteoporotic fractures: a systematic review of US healthcare costs and resource utilization. Pharmacoeconomics 30:147–170
Konnopka A, Jerusel N, Konig HH (2009) The health and economic consequences of osteopenia- and osteoporosis-attributable hip fractures in Germany: estimation for 2002 and projection until 2050. Osteoporos Int 20:1117–1129
Häussler B, Gothe H, Gol D, Glaeske G, Pientka L, Felsenberg D (2007) Epidemiology, treatment and costs of osteoporosis in Germany—the BoneEVA Study. Osteoporos Int 18:77–84
Schwenkglenks M, Lippuner K, Hauselmann HJ, Szucs TD (2005) A model of osteoporosis impact in Switzerland 2000–2020. Osteoporos Int 16:659–667
Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A (2007) Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res 22:465–475
Burge RT, Worley D, Johansen A, Bhattacharyya S, Bose U (2001) The cost of osteoporotic fractures in the UK: projections for 2000–2020. J Med Econ 4:51–62
Tosteson AN, Jonsson B, Grima DT, O’Brien BJ, Black DM, Adachi JD (2001) Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int 12:849–857
Statistisches Bundesamt (2006) Generationensterbetafeln für Deutschland. Modellrechnungen für die Geburtsjahrgänge 1871–2004 [Generation life tables for Germany. A model calculation for the birth cohorts 1871–2004]. Statistisches Bundesamt, Wiesbaden
Jerusel N (2009) Analyse und Prognose der Krankheitslast Osteoporose—attributabler proximaler Femurfrakturen in Deutschland [Analysis and Prognosis of the health burden of osteoporosis attributable proximal femur fractures in Germany]. University of Leipzig, Dissertation
Wildner M (2001) Osteoporose [Osteoporosis]. Dtsch med Wochenschr 126:A1170–A1172
Konig HH, Barry JC (2004) Cost–utility analysis of orthoptic screening in kindergarten: a Markov model based on data from Germany. Pediatrics 113:e95–e108
Paggiosi M, Glueer C, Roux C, Reid D, Felsenberg D, Barkmann R, Eastell R (2011) International variation in proximal femur bone mineral density. Osteoporos Int 22:721–729
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Statistisches Bundesamt (2011) Tiefgegliederte Diagnosedaten der Krankenhauspatientinnen und -patienten 2009 [Deep stratified diagnosis—data from female and male inpatients 2009]. Statistisches Bundesamt, Wiesbaden
Gedeborg R, Engquist H, Berglund L, Michaelsson K (2008) Identification of incident injuries in hospital discharge registers. Epidemiology 19:860–867
Icks A, Haastert B, Wildner M, Becker C, Meyer G (2008) Trend of hip fracture incidence in Germany 1995–2004: a population-based study. Osteoporos Int 19:1139–1145
Finnern HW, Sykes DP (2003) The hospital cost of vertebral fractures in the EU: estimates using national datasets. Osteoporos Int 14:429–436
Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J (2000) Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 15:1384–1392
Einsiedel T, Becker C, Stengel D, Schmelz A, Kramer M, Daxle M, Lechner F, Kinzl L, Gebhard F (2006) Do injuries of the upper extremity in geriatric patients end up in helplessness? A prospective study for the outcome of distal radius and proximal humerus fractures in individuals over 65. Z Gerontol Geriatr 39:451–461
Boufous S, Finch C, Close J, Day L, Lord S (2007) Hospital admissions following presentations to emergency departments for a fracture in older people. Inj Prev 13:211–214
Van Staa TP, Leufkens HG, Cooper C (2002) Does a fracture at one site predict later fractures at other sites? A British cohort study. Osteoporos Int 13:624–629
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Kanis JA, Borgstrom F, Johnell O, Jonsson B (2004) Cost-effectiveness of risedronate for the treatment of osteoporosis and prevention of fractures in postmenopausal women. Osteoporos Int 15:862–871
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Konig HH, Bernert S, Angermeyer MC (2005) Health Status of the German population: results of a representative survey using the EuroQol questionnaire. Gesundheitswesen 67:173–182
Dolan P (1997) Modeling valuations for EuroQol health states. Med Care 35:1095–1108
Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, Jonsson B (2004) The risk and burden of vertebral fractures in Sweden. Osteoporos Int 15:20–26
Institut für das Entgeltsystem im Krankenhaus (INEK) (2011) G-DRG V2010 Browser. Institut für das Entgeltsystem im Krankenhaus, Siegburg
Deutsche Krankenhausgesellschaft (2011) Landesbasisfallwerte der Bundesländer 2005–2011 [Base rates of the German Federal States 2005–2011]. http://www.dkgev.de/media/file/7827.LBFW_2005_2010_Stand_160610.pdf. Accessed 18 Feb 2012
Krauth C, Hessel F, Hansmeier T, Wasem J, Seitz R, Schweikert B (2005) Empirical standard costs for health economic evaluation in Germany—a proposal by the working group methods in health economic evaluation. Gesundheitswesen 67:736–746
Kreck S, Klaus J, Leidl R, von Tirpitz C, Konnopka A, Matschinger H, Konig HH (2008) Cost effectiveness of ibandronate for the prevention of fractures in inflammatory bowel disease-related osteoporosis: cost-utility analysis using a Markov model. Pharmacoeconomics 26:311–328
Kassenärztliche Bundesvereinigung (KBV) (2010) Einheitlicher Bewertungsmaßstab (EBM) 2010. http://www.kbv.de/ebm2012/EBMGesamt.htm. Accessed 18 Feb 2012
Rote Liste Service GmbH (2009) Rote Liste 2009 [Red List 2009]. Rote Liste Service GmbH, Frankfurt am Main
Statistisches Bundesamt (2011) Pflegestatistik 2009. Pflege im Rahmen der Pflegeversicherung. Deutschlandergebnisse [Statistics on care 2009—care in the context of the care insurance results from Germany]. Statistisches Bundesamt, Wiesbaden
Bundesverband AOK (2009) Krankheitsartenstatistik 2008 [Disease statistic 2008]. AOK Bundesverband, Berlin
Deutsche Rentenversicherung (2010) Statistik der Deutschen Rentenversicherung. Rehabilitation 2009 Band 179 [Statistic of the German pension insurance fund—rehabilitation 2009 volume 179]. Deutsche Rentenversicherung, Berlin
Statistisches Bundesamt (2011) Verdienste und Arbeitskosten, Jahr 2010, Fachserie 16 Reihe 2.3 [Earning and labour cost 2010, Series 16(2.3)]. Statistisches Bundesamt, Wiesbaden
Statistisches Bundesamt (2009) Mikrozenus 2009, Fachserie 1 Reihe 4.1.1 [Microcensus 2009, Series 1(4.1.1)]. Statistisches Bundesamt, Wiesbaden
Statistisches Bundesamt (2003) Wo bleibt die Zeit? Die Zeitverwendung der Bevölkerung in Deutschland 2001/02 [Where has the time gone? The time use of the German population in 2001/02]. Statistisches Bundesamt, Wiesbaden
Briggs A, Claxton K, Sculpher M (2007) Decision modelling for health economic evaluation. Oxford University, Oxford
Statistisches Bundesamt (2012) Die Gesundheitsberichterstattung des Bundes: Ausgaben, Kosten, Finanzierung [Federal health monitoring: expenditures, costs and financing].https://www.gbebund.de/gbe10/abrechnung.prc_abr_test_logon?p_uid=gast&p_aid=51115479&p_sprache=E&p_knoten=TR19200. Accessed 15 April 2012
Nguyen ND, Center JR, Eisman JA, Nguyen TV (2007) Bone loss, weight loss, and weight fluctuation predict mortality risk in elderly men and women. J Bone Miner Res 22:1147–1154
Melton LJ 3rd, Thamer M, Ray NF, Chan JK, Chesnut CH 3rd, Einhorn TA, Johnston CC, Raisz LG, Silverman SL, Siris ES (1997) Fractures attributable to osteoporosis: report from the National Osteoporosis Foundation. J Bone Miner Res 12:16–23
Mackey DC (2011) Determining which fractures are osteoporotic: empirical associations would provide better evidence than expert opinion. J Clin Epidemiol 64:45
Cooper C, Cole ZA, Holroyd CR, Earl SC, Harvey NC, Dennison EM, Melton LJ, Cummings SR, Kanis JA (2011) Secular trends in the incidence of hip and other osteoporotic fractures. Osteoporos Int 22:1277–1288
Oxford Centre of Evidence-Based Medicine (2009) Levels of evidence (March 2009). http://www.cebm.net/index.aspx?o=1025. Accessed 18 Feb 2012
Mueller D, Gandjour A (2009) Cost-effectiveness of using clinical risk factors with and without DXA for osteoporosis screening in postmenopausal women. Value Health 12:1106–1117
Kennedy CC, Papaioannou A, Adachi JD (2006) Treating osteoporosis: economic aspects of bisphosphonate therapy. Expert Opin Pharmacother 7:1457–1467
Cummings SR, Melton LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767
Becker C, Cameron ID, Klenk J, Lindemann U, Heinrich S, Konig HH, Rapp K (2011) Reduction of femoral fractures in long-term care facilities: the Bavarian fracture prevention study. PLoS One 6:e24311
Cameron ID, Murray GR, Gillespie LD, Robertson MC, Hill KD, Cumming RG, Kerse N (2010) Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev 20:CD005465
Gillespie LD, Robertson MC, Gillespie WJ, Lamb SE, Gates S, Cumming RG, Rowe BH (2009) Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev CD007146
Davis JC, Robertson MC, Ashe MC, Liu-Ambrose T, Khan KM, Marra CA (2009) Does a home-based strength and balance programme in people aged > or =80 years provide the best value for money to prevent falls? A systematic review of economic evaluations of falls prevention interventions. Br J Sports Med 44:80–89
Church J, Goodall S, Norman R, Haas M (2011) An economic evaluation of community and residential aged care falls prevention strategies in NSW. N S W Public Health Bull 22:60–68
Gandjour A, Weyler EJ (2008) Cost-effectiveness of preventing hip fractures by hip protectors in elderly institutionalized residents in Germany. Value Health 11:1088–1095
Acknowledgments
This work was funded by the German Federal Ministry of Education and Research (BMBF), Germany, FKZ:01EC1007C. Petra Benzinger and Kilian Rapp were supported by a grant from the “Forschungskolleg Geriatrie” of the Robert Bosch Foundation. The BMBF and the Robert Bosch Foundation had no further role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 309 kb)
Rights and permissions
About this article
Cite this article
Bleibler, F., Konnopka, A., Benzinger, P. et al. The health burden and costs of incident fractures attributable to osteoporosis from 2010 to 2050 in Germany—a demographic simulation model. Osteoporos Int 24, 835–847 (2013). https://doi.org/10.1007/s00198-012-2020-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-012-2020-z