Abstract
Introduction
Risedronate, a bisphosphonate for treatment and prevention of osteoporosis, has been shown in several clinical trials to reduce the risk of fractures in postmenopausal women with osteoporosis. The cost-effectiveness of risedronate treatment has previously been evaluated within different country settings using different model and analysis approaches. The objective of this study was to assess the cost-effectiveness of risedronate in postmenopausal women in four European countries—Sweden, Finland, Spain, and Belgium—by making use of the same modelling framework and analysis setup.
Methods
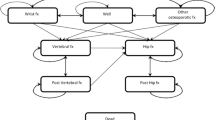
A previously developed Markov cohort model for the evaluation of osteoporosis treatments was used to estimate the cost-effectiveness of risedronate treatment. For each country, the model was populated with local mortality, fracture incidence, and cost data. Hip fractures, clinical vertebral fractures, and wrist fractures were included in the model.
Results
The incremental cost per quality-adjusted life years (QALY) gained from a 5-year intervention with risedronate compared to “no intervention” in 70-year-old women at the threshold of osteoporosis [T-score = −2.5 based on National Health and Nutrition Examination Survey (NHANES) III data] and previous vertebral fracture was estimated to be €860, €19,532, €11,782, and €32,515 in Sweden, Finland, Belgium, and Spain, respectively. Among 70-year-old women at the threshold of osteoporosis without previous fracture the estimated cost per QALY gained ranged from €21,148 (Sweden) to €80,100 (Spain). The differences in cost-effectiveness between countries are mainly explained by different costs (fracture and treatment costs), fracture risks, and discount rates. Based on cost per QALY gained threshold values found in the literature, the study results indicated risedronate to be cost effective in the treatment of elderly women with established osteoporosis in all the included countries.
Conclusions
At a hypothetical threshold value of €40,000 per QALY gained, the results in this study indicate that risedronate is a cost-effective treatment in elderly women at the threshold of osteoporosis (i.e., a T-score of −2.5) with prevalent vertebral fractures in Sweden, Finland, Belgium, and Spain.






Similar content being viewed by others
References
Cummings SR, Melton, LJ (2002) Epidemiology and outcomes of osteoporotic fractures. Lancet 359:1761–1767
Report on Osteoporosis in the European Community - Action for Prevention (1998). European Commission – Employment & Social Affairs
Kanis JA, Johnell O (2005) Requirements for DXA for the management of osteoporosis in Europe. Osteoporos Int 16:229–238
Delmas PD (2002) Treatment of postmenopausal osteoporosis. Lancet 359:2018–2026
Reginster J, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R (2000) Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int 11:83–91
Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD (1999) Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA 282:1344–1352
McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY (2001) Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 344:333–340
Drummond M, O’Brien B, Stoddart G, Torrance G (1997) Methods for the economic evaluation of health care programmes. Oxford University Press, Oxford
Kanis JA, Borgstrom F, Johnell O, Jonsson B (2004) Cost-effectiveness of risedronate for the treatment of osteoporosis and prevention of fractures in postmenopausal women. Osteoporos Int 15:862–871
Borgstrom F, Zethraeus N (2003) Economic assessment based on a clinical study of risedronate. Fracture prevention in elderly women with osteoporosis is cost-effective. Lakartidningen 100:36–40
Iglesias CP, Torgerson DJ, Bearne A, Bose U (2002) The cost utility of bisphosphonate treatment in established osteoporosis. Quart J Med 95:305–311
Hart WM, Rubio-Terres C, Burrell A, Aristegui I, Escobar-Jimenez Y (2002) Análisis farmacoeconómico del tratamiento de la osteoporosis postmenopáusica con risedronato o alendronato. REEMO 11:97–104
Brecht JG, Kruse HP, Felsenberg D, Mohrke W, Oestreich A, Huppertz E (2003) Pharmacoeconomic analysis of osteoporosis treatment with risedronate. Int J Clin Pharmacol Res 23:93–105
Sonnenberg FA, Beck JR (1993) Markov models in medical decision making: a practical guide. Med Decis Making 13:322–338
Jonsson B, Christiansen C, Johnell O, Hedbrandt J, Karlsson G (1996) Cost-effectiveness of fracture prevention in established osteoporosis. Scand J Rheumatol Suppl 103:30–38
Johnell O, Jonsson B, Jonsson L, Black D (2003) Cost effectiveness of alendronate (Fosamax) for the treatment of osteoporosis and prevention of fractures. Pharmacoeconomics 21:305–314
Jonsson L, Borgstrom F, Zethraeus N (2003) Cost-effectiveness of alendronate treatment of osteoporosis in Denmark. An economic evaluation based on the Fracture Intervention Trial. Ugeskr Laeger 165:4112–4116
Zethraeus N, Johannesson M, Jonsson B (1999) A computer model to analyze the cost-effectiveness of hormone replacement therapy. Int J Technol Assess Health Care 15:352–365
Kanis JA, Dawson A, Oden A, Johnell O, de Laet C, Jonsson B (2001) Cost-effectiveness of preventing hip fracture in the general female population. Osteoporos Int 12:356–361
Kanis JA, Johnell O, Oden A, De Laet C, Oglesby A, Jonsson B (2002) Intervention thresholds for osteoporosis. Bone 31:26–31
Kanis JA, Johnell O, Oden A, Jonsson B, Dawson A, Dere W (2000) Risk of hip fracture derived from relative risks: an analysis applied to the population of Sweden. Osteoporos Int 11:120–127
Tosteson AN, Jonsson B, Grima DT, O’Brien BJ, Black DM, Adachi JD (2001) Challenges for model-based economic evaluations of postmenopausal osteoporosis interventions. Osteoporos Int 12:849–857
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmö. Osteoporos Int 11:669–674
Kannus P, Niemi S, Parkkari J, Palvanen M, Vuori I, Jarvinen M (1999) Hip fractures in Finland between 1970 and 1997 and predictions for the future. Lancet 353:802–805
Federaal Ministerie van Sociale Zaken, Volksgezondheid en Leefmilieu. Bestuur van de gezondheidszorgen. Commissie voor toezicht op en evaluatie van statistische gegevens die verband houden met de medische activiteiten in de ziekenhuizen. Rijksadministratif centrum. Brussel. Upon written request
Statistiek NIvd. NIS-Dienst Verspreiding, Leuvenseweg 44, B-1000 Brussel. Upon written request
Elffors I, Allander E, Kanis JA, Gullberg B, Johnell O, Dequeker J, Dilsen G, Gennari C, Lopes Vaz AA, Lyritis G et al. (1994) The variable incidence of hip fracture in southern Europe: the MEDOS Study. Osteoporos Int 4:253–263
Johnell O, Gullberg B, Kanis JA (1997) The hospital burden of vertebral fracture in Europe: a study of national register sources. Osteoporos Int 7:138–144
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. Br Med J 312:1254–1259
Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, Johnston CC Jr, Lindsay R (1998) Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int 8:468–489
Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA 3rd, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, Eisman J, Fujiwara S, Garnero P, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) A meta-analysis of previous fracture and subsequent fracture risk. Bone 35:375–382
Cranney A, Tugwell P, Adachi J, Weaver B, Zytaruk N, Papaioannou A, Robinson V, Shea B, Wells G, Guyatt G (2002) Meta-analyses of therapies for postmenopausal osteoporosis. III. Meta-analysis of risedronate for the treatment of postmenopausal osteoporosis. Endocr Rev 23:517–523
Tonino RP, Meunier PJ, Emkey R, Rodriguez-Portales JA, Menkes CJ, Wasnich RD, Bone HG, Santora AC, Wu M, Desai R, Ross PD (2000) Skeletal benefits of alendronate: 7-year treatment of postmenopausal osteoporotic women. Phase III Osteoporosis Treatment Study Group. J Clin Endocrinol Metab 85:3109–3115
Stock JL, Bell NH, Chesnut CH 3rd, Ensrud KE, Genant HK, Harris ST, McClung MR, Singer FR, Yood RA, Pryor-Tillotson S, Wei L, Santora AC 2nd (1997) Increments in bone mineral density of the lumbar spine and hip and suppression of bone turnover are maintained after discontinuation of alendronate in postmenopausal women. Am J Med 103:291–297
Watts N, Barton I, Olszynski W, McKeever C, McClung M, Grauer A (2005) Sustained reduction of vertebral fracture risk in the year after discontinuation of risedronate treatment. Osteoporos Int 16:[Abstract s40]
Statistical Yearbook of Sweden 2004
Läkemedelsförmånsnämndens allmänna råd (2003). Stockholm
Rovira J, Antonanzas F (1995) Economic analysis of health technologies and programmes. A Spanish proposal for methodological standardisation. Pharmacoeconomics 8:245–252
International Society for Pharmacoeconomics & Outcomes Research. Pharmacoeconomic Guidelines Around The World. http://www.ispor.org/PEguidelines/countrydet.asp?c=7&t=1. Access date: 2005-08-22
A proposal for methodological guidelines for economic evaluation of pharmaceuticals (1995) Belgian Society for Pharmacoepidemiology, Brussels
Kanis JA, Oden A, Johnell O, Jonsson B, de Laet C, Dawson A (2001) The burden of osteoporotic fractures: a method for setting intervention thresholds. Osteoporos Int 12:417–427
Instituto Nacional de Estadistica. Tablas de Mortalidad de la Población de España 1998–1999. http://www.ine.es/inebase/cgi/um?M=%2Ft20%2Fp319%2Fa1998%2F&O=pcaxis&N=&L=1. Access date: 2005-08-22
Statistical Yearbook of Finland (2003). Statistics Finland, Helsinki
Scientific Institute of Public Health, Unit of Epidemiology. Overall Mortality: Number of Deaths, Crude Rate (/100000): Belgium 1997: Females. http://www.iph.fgov.be/epidemio/spma. Access date: 2004-04-22
Statistical Yearbook of Sweden (2004). Statistics Sweden, Stockholm
Oden A, Dawson A, Dere W, Johnell O, Jonsson B, Kanis JA (1998) Lifetime risk of hip fractures is underestimated. Osteoporos Int 8:599–603
Johnell O, Kanis JA, Oden A, Sernbo I, Redlund-Johnell I, Petterson C, De Laet C, Jonsson B (2004) Mortality after osteoporotic fractures. Osteoporos Int 15:38–42
Kanis JA, Oden A, Johnell O, De Laet C, Jonsson B, Oglesby AK (2003) The components of excess mortality after hip fracture. Bone 32:468–473
Parker MJ, Anand JK (1991) What is the true mortality of hip fractures? Public Health 105:443–446
Cooper C, Atkinson EJ, Jacobsen SJ, O’Fallon WM, Melton LJ 3rd (1993) Population-based study of survival after osteoporotic fractures. Am J Epidemiol 137:1001–1005
Cauley JA, Thompson DE, Ensrud KC, Scott JC, Black D (2000) Risk of mortality following clinical fractures. Osteoporos Int 11:556–561
Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA (1999) Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet 353:878–882
Lundberg L, Johannesson M, Isacson D, Borgquist L (1999) Health state utilities in a general population in relation to age, gender and socioeconomic factors. Eur J Public Health 9:211–217
Kind P, Hardman G, Macron S, UK Population Norms for EQ-5D (1999) Centre for Health Economics, University of York, York
Ohinmaa A, Sintonen H (1996) Quality of life of the Finnish population as measured by the EuroQol. Institut Universitari de Salut Publica de Catalunya, Barcelona
Dolan P (1997) Modeling valuations for EuroQol health states. Med Care 35:1095–1108
Zethraeus N, Borgström F, Johnell O, Kanis J, Jönsson B (2002) Costs and quality of life associated with osteoporosis related fractures - results from a Swedish survey. Working Paper Series in Economics and Finance, 512
Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, Jonsson B (2004) The risk and burden of vertebral fractures in Sweden. Osteoporos Int 15:20–26
Brazier JE, Green C, Kanis JA (2002) A systematic review of health state utility values for osteoporosis-related conditions. Osteoporos Int 13:768–776
National Osteoporosis Foundation (1998) Osteoporosis: review of the evidence for prevention, diagnosis and treatment and cost-effective analysis. Osteoporos Int 8(Suppl 4):1–88
Gabriel SE, Kneeland TS, Melton LJ 3rd, Moncur MM, Ettinger B, Tosteson AN (1999) Health-related quality of life in economic evaluations for osteoporosis: whose values should we use? Med Decis Making 19:141–148
Cranny A, Coyle D, Wells G, Jolly E, Tugwell P (2001) The psychometric properties of patient preferences in osteoporosis. J Rheumatology 28:132–137
Merlino LA, Bagchi I, Taylor TN, Utrie P, Chrischilles E, Sumner DR, Mudano A, Saag KG (2001) Preferences for fractures and other glucocorticoid-associated adverse events among rheumatoid arthritis patients. Med Decis Making 21:122–132
Hallberg I, Rosenqvist AM, Kartous L, Lofman O, Wahlstrom O, Toss G (2004) Health-related quality of life after osteoporotic fractures. Osteoporos Int 15:834–841
Oleksik A, Lips P, Dawson A, Minshall ME, Shen W, Cooper C, Kanis J (2000) Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J Bone Miner Res 15:1384–1392
Tosteson AN, Gabriel SE, Grove MR, Moncur MM, Kneeland TS, Melton LJ 3rd (2001) Impact of hip and vertebral fractures on quality-adjusted life years. Osteoporos Int 12:1042–1049
Hall SE, Criddle RA, Comito TL, Prince RL (1999) A case-control study of quality of life and functional impairment in women with long-standing vertebral osteoporotic fracture. Osteoporos Int 9:508–515
Jonsson B, Christiansen C, Johnell O, Hedbrandt J, Karlsson G (1996) Cost-effectiveness of fracture prevention in established osteoporosis. Scand J Rheumatol 103(Suppl):30–38; discussion 39–40
Ekman M, Zethraeus N, Dahlstrom U, Hoglund C (2002) Cost-effectiveness of bisoprolol in chronic heart failure. Lakartidningen 99:646–650
Sacristan JA, Oliva J, Del Llano J, Prieto L, Pinto JL (2002) What is an efficient health technology in Spain? Gac Sanit 16:334–343
Raftery J (2001) NICE: faster access to modern treatments? Analysis of guidance on health technologies. Br Med J 323:1300–1303
Kanis JA, Jonsson B (2002) Economic evaluation of interventions for osteoporosis. Osteoporos Int 13:765–767
Macroeconomics and Health: investing in health for economic development. Report of the Commission on Macroeconomics and Health (2001) Geneva
Hart W, Rubio-Terres C, Burrell A, Aristegui I, Escobar-Jimenez Y (2002) Análisis farmacoeconómico del tratamiento de la osteoporosis postmenopáusica con risedronato o alendronato. REEMO 11
Kanis JA, Johnell O, Oden A, Sembo I, Redlund-Johnell I, Dawson A, De Laet C, Jonsson B (2000) Long-term risk of osteoporotic fracture in Malmo. Osteoporos Int 11:669–674
Zethraeus N, Stromberg L, Jonsson B, Svensson O, Ohlen G (1997) The cost of a hip fracture. Estimates for 1,709 patients in Sweden. Acta Orthop Scand 68:13–17
Sintonen H, Vokkolainen A, Alhava E (2002) Osteoporoottisten lonkkamurtumien taloudelliset ja elämänlaadulliset seuraukset. Stakes Aiheita 34–35
Autier P, Haentjens P, Bentin J, Baillon JM, Grivegnee AR, Closon MC, Boonen S (2000) Costs induced by hip fractures: a prospective controlled study in Belgium. Belgian Hip Fracture Study Group. Osteoporos Int 11:373–380
StadsledningskontoretsRedovisningsstab (2003) Stockholms stads budgetavräkning 2002. Stadsledningskontorets Redovisningsstab, Stockholm
Hujanen T (2003) Terveydenhuollon yksikkökustannukset Suomessa vuonna 2001 Stakes Aiheita 1/2003, Helsinki
Belgian Social Security System. http://www.riziv.be/insurer/fr/rate/pdf/2004/doctors/raad-20041001-fr.pdf. Access date: 2005-07-02
Honorato J, Gomez-Outes A, Navarro-Quilis A, Martinez-Gonzalez J, Rocha E, Planes A (2004) Pharmacoeconomic analysis of bemiparin and enoxaparin as prophylaxis for venous thromboembolism in total knee replacement surgery. Pharmacoeconomics 22:885–894
The Swedish Association of the Pharmaceutical Industry. http://www.fass.se/LIF/produktfakta/artikel_produkt.jsp?NplID=19991007000217&DocTypeID=7&UserTypeID=2. Access date: 2005-05-06
Yliopiston Apteekki. http://www.yliopistonapteekki.fi/searchProductInfo.do?searchResultByProductName=OPTINATE+TABLETTI+5MG&manufacturer=AVENTI. Access date: 2005-05-03
Centre Belge d´Information Pharmacothérapeutique. http://www.cbip.be/ggr/index.cfm?ggrWelk=/nindex/ggr/stof/IN_R.cfm. Access date: 2005-07-25
Base de datos medicamentos. http://pfarmals.portalfarma.com/default.asp. Access date: 2005-07-26
Priser för södra sjukvårdsregionen - Vårdtjänster per klinik (2003) Universitetssjukhuset i Lund
Osteoporosis in the European Community: A Call to Action: An audit of policy developments since 1998 (2001) International Osteoporosis Foundation
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial support was obtained via an unrestricted grant from The Alliance for Better Bone Health.
Rights and permissions
About this article
Cite this article
Borgström, F., Carlsson, Å., Sintonen, H. et al. The cost-effectiveness of risedronate in the treatment of osteoporosis: an international perspective. Osteoporos Int 17, 996–1007 (2006). https://doi.org/10.1007/s00198-006-0094-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-006-0094-1