Abstract
Purpose
Mechanical stimulation plays an important role in the development and remodelling of tendons. The aim of the study was to evaluate the effects of mechanical stimulation on the expression of extracellular matrix proteins in human primary rotator cuff (RC) fibroblasts.
Methods
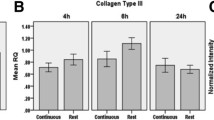
RC fibroblasts were isolated from patients with degenerative RC tears and characterized using flow cytometry and immunohistochemistry. Cells were stimulated using the Flexcell FX5K™ Tension System. The stimulation regime was a uniaxial sinusoidal waveform with 10 % elongation and a frequency of 0.5 Hz, whereby each cycle consists of 10-s strain and 30-s relaxation. Data were normalized to mechanically unstimulated control groups for every experimental condition. RT-qPCR was performed to determine relative mRNA levels, and collagen production was measured by a colorimetric assay.
Results
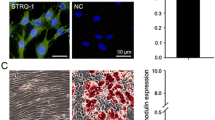
The positive expression of CD91 and CD10, and negativity for CD45 and CD4 confirmed the fibroblast phenotype of RC primary cells. RT-qPCR revealed that 10 % continuous cyclic strain for 7 and 14 days induced a significant increase in the mRNA expression both on the matrix metalloproteinases MMP1, MMP3, MMP13, and MMP14 and on the extracellular matrix proteins decorin, tenascin-C, and scleraxis. Furthermore, mechanically stimulated groups produced significantly higher amounts of total collagen.
Conclusion
These results may contribute to a better understanding of strain-induced tendon remodelling and will form the basis for the correct choice of applied force in rehabilitation after orthopaedic surgery. These findings underline the fact that early passive motion of the joint in order to induce remodelling of the tendon should be included within a rehabilitation protocol for rotator cuff repair.





Similar content being viewed by others
References
Archambault J, Tsuzaki M, Herzog W, Banes AJ (2002) Stretch and interleukin-1beta induce matrix metalloproteinases in rabbit tendon cells in vitro. J Orthop Res 20(1):36–39
Chokalingam K, Juncosa-Melvin N, Hunter SA, Gooch C, Frede C, Florert J et al (2009) Tensile stimulation of murine stem cell collagen sponge constructs increases collagen type I gene expression and linear stiffness. Tissue Eng Part A 15:2561–2570
Halper J, Kjaer M (2014) Basic components of connective tissues and extracellular matrix: elastin, fibrillin, fibulins, fibrinogen, fibronectin, laminin, tenascins and thrombospondins. Adv Exp Med Biol 802:31–47
Hromadnikova I, Nguyen TT, Zlacka D, Sedlackova L, Popelka S, Veigl D, Pech J, Vavrincova P, Sosna A (2008) Expression of heat shock protein receptors on fibroblast-like synovial cells derived from rheumatoid arthritis-affected joints. Rheumatol Int 28(9):837–844
Huisman E, Lu A, McCormack RG, Scott A (2014) Enhanced collagen type I synthesis by human tenocytes subjected to periodic in vitro mechanical stimulation. BMC Musculoskelet Disord 15:386
Jung HJ, Fisher MB, Woo SL (2009) Role of biomechanics in the understanding of normal, injured, and healing ligaments and tendons. Sports Med Arthrosc Rehabil Ther Technol 1(1):9–26
Kjaer M (2004) Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev 84(2):649–698
Pins GD, Christiansen DL, Patel R, Silver FH (1997) Self-assembly of collagen fibers. Influence of fibrillar alignment and decorin on mechanical properties. Biophys J 73(4):2164–2172
Popov C, Burggraf M, Kreja L, Ignatius A, Schieker M, Docheva D (2015) Mechanical stimulation of human tendon stem/progenitor cells results in upregulation of matrix proteins, integrins and MMPs, and activation of p38 and ERK1/2 kinases. BMC Mol Biol 16:6
Rhee YG, Cho NS, Yoo JH (2014) Clinical outcome and repair integrity after rotator cuff repair in patients older than 70 years versus patients younger than 70 years. Arthroscopy 30(5):546–554
Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P (2012) Initial Achilles tendon repair strength-synthesized biomechanical data from 196 cadaver repairs. Int Orthop 36:1947–1951
Sadoghi P, Rosso C, Valderrabano V, Leithner A, Vavken P (2013) Platelets for treatment of Achilles tendon injuries. J Orthop Res 31:111–118
Sher JS, Uribe JW, Posada A, Murphy BJ, Zlatkin MB (1995) Abnormal findings on magnetic resonance images of asymptomatic shoulders. J Bone Joint Surg Am 77(1):10–15
Tanzer ML (1973) Cross-linking of collagen. Science 180(86):561–566
Sun HB, Yokota H (2002) Reduction of cytokine-induced expression and activity of MMP-1 and MMP-13 by mechanical strain in MH7A rheumatoid synovial cells. Matrix Biol 21(3):263–270
Silver FH, Freeman JW, Seehra GP (2003) Collagen self-assembly and the development of tendon mechanical properties. J Biomech 36:1529–1553
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N et al (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3(7):1–12
Vavken P, Murray MM (2010) Translational studies in anterior cruciate ligament repair. Tissue Eng Part B Rev 16:5–11
Vogel KG, Heinegard D (1985) Characterization of proteoglycans from adult bovine tendon. J Biol Chem 260(16):9298–9306
Wang JH (2006) Mechanobiology of tendon. J Biomech 39(9):1563–1582
Wang T, Lin Z, Day RE, Gardiner B, Landao-Bassonga E, Rubenson J (2013) Programmable mechanical stimulation influences tendon homeostasis in a bioreactor system. Biotechnol Bioeng 110(5):1495–1507
Wieser K, Farshad M, Meyer DC, Conze P, von Rechenberg B, Gerber C (2015) Tendon response to pharmaco-mechanical stimulation of the chronically retracted rotator cuff in sheep. Knee Surg Sports Traumatol Arthrosc 23(2):577–584
Woo SLY, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC et al (1980) The biomechanical and biochemical properties of swine tendons—long term effects of exercise on the digital extensors. Connect Tissue Res 7:177–183
Woo SL, Fisher MB, Feola AJ (2008) Contribution of biomechanics to management of ligament and tendon injuries. Mol Cell Biomech 5(1):49–68
Wren TA, Yerby SA, Beaupre GS, Carter DR (2001) Mechanical properties of the human Achilles tendon. Clin Biomech 16:245–251
Yamamoto E, Kogawa D, Tokura S, Hayashi K (2005) Effects of the frequency and duration of cyclic stress on the mechanical properties of cultured collagen fascicles from the rabbit patellar tendon. J Biomech Eng 127:1168–1175
Yang G, Im HJ, Wang JH (2005) Repetitive mechanical stretching modulates IL-1beta induced COX-2, MMP-1 expression, and PGE2 production in human patellar tendon fibroblasts. Gene 363:166–172
Zhang J, Wang JH (2013) The effects of mechanical loading on tendons—an in vivo and in vitro model study. PLoS ONE 8(8):e71740
Acknowledgments
This study was supported by the Austrian Society of Orthopaedic Surgery Research Grant 2013 and the Medical University of Graz. No commercial benefits of any kind have been or will be received from institutions related directly or indirectly to the subject of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Lohberger, B., Kaltenegger, H., Stuendl, N. et al. Impact of cyclic mechanical stimulation on the expression of extracellular matrix proteins in human primary rotator cuff fibroblasts. Knee Surg Sports Traumatol Arthrosc 24, 3884–3891 (2016). https://doi.org/10.1007/s00167-015-3790-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00167-015-3790-6