Abstract
Purpose
To investigate whether neuromuscular blocking agents (NMBA) exert beneficial effects in acute respiratory distress syndrome (ARDS) by reason of their action on respiratory mechanics, particularly transpulmonary pressures (P L).
Methods
A prospective randomised controlled study in patients with moderate to severe ARDS within 48 h of the onset of ARDS. All patients were monitored by means of an oesophageal catheter and followed up for 48 h. Moderate ARDS patients were randomised into two groups according to whether they were given a 48-h continuous infusion of cisatracurium besylate or not (control group). Severe ARDS patients did not undergo randomisation and all received cisatracurium besylate per protocol. The changes during the 48-h study period in oxygenation and in respiratory mechanics, including inspiratory and expiratory P L and driving pressure, were assessed and compared. Delta P L (∆P L) was defined as inspiratory P L minus expiratory P L.
Results
Thirty patients were included, 24 with moderate ARDS and 6 with severe ARDS. NMBA infusion was associated with an improvement in oxygenation in both moderate and severe ARDS, accompanied by a decrease in both plateau pressure and total positive end-expiratory pressure. The mean inspiratory and expiratory P L were higher in the moderate ARDS group receiving NMBA than in the control group. In contrast, there was no change in either driving pressure or ∆P L related to NMBA administration.
Conclusions
NMBA could exert beneficial effects in patients with moderate ARDS, at least in part, by limiting expiratory efforts.
Similar content being viewed by others
Introduction
Most of the recent advances in the treatment of acute respiratory distress syndrome (ARDS) are focused on mechanical ventilation with low tidal volume [1], moderate to high level of positive end-expiratory pressure (PEEP) [2] and prolonged prone positioning [3]. Numerous pharmacological interventions have been associated with disappointing results including intravenous [4–7] and inhaled molecules [8–10]. The only conclusive positive pharmacological study in ARDS was the use of a short infusion period of cisatracurium besylate [11]. The mechanisms explaining how cisatracurium besylate improves the outcome are still unclear and maybe multiple [12]. Besides a proven anti-inflammatory effect of the molecule itself [13, 14] through the nicotinic acetylcholine pathway, it has been advocated that neuromuscular blocking agents (NMBA) could lead to more gentle mechanical ventilation with better synchronism between the patient and ventilator and a more homogenous distribution of the pressurization during tidal ventilation [12]. In ARDS patients, the optimal way to ensure mechanical ventilation is still debated. On the one hand, one could argue that preserving spontaneous ventilation during the acute phase of ARDS may be beneficial, notably by preventing the risk of diaphragmatic dysfunction [15]. On the other hand, spontaneous breathing efforts in the most severe forms of ARDS could cause uncontrolled switches in transpulmonary pressure (P L) increase despite limited plateau pressure (Pplat), at least in animal models [16]. The main objective of the present prospective randomised controlled study was therefore to assess the effects of a 48-h infusion period of NMBA on respiratory mechanics (Pplat, total PEEP, driving pressure, inspiratory and expiratory P L and ∆P L) in moderate to severe ARDS. The secondary objective was to assess and compare the percentages of positive expiratory P L during the 48 h of the study.
Methods
This prospective randomised study was conducted in two ICUs in a university teaching hospital in Marseille, France. This registered (NCT01573715) study was approved by the ethics committee (Comité de Protection des Personnes Sud Méditerranée V) and the French national authority (Agence Nationale de Sécurité du Médicament). Importantly, the ethics committee asked us to use NMBA in all severe ARDS patients and to randomise only the patients presenting with a PaO2/FiO2 greater than 100. Written informed consent was obtained from a relative of the patient. If patients were able to read the leaflet at some point after inclusion in the study, they were approached to confirm participation in the trial.
Patients
All intubated and mechanically ventilated patients were included if they presented with moderate to severe ARDS [17] with a PaO2/FiO2 less than 150 with PEEP at least 5 within the first 48 h of the onset of ARDS. Exclusion criteria included age less than 18, pregnancy, patient receiving continuous infusion of NMBA, known NMBA allergy, contraindication to introduction of nasogastric tube, undrained pneumothorax, treatment with extracorporeal membrane oxygenation or extracorporeal CO2 removal, increased intracranial pressure, respiratory chronic insufficiency, body mass index greater than 40 kg/m2, severe chronic liver disease (Child–Pugh class C), bone marrow transplantation or chemotherapy-induced neutropenia, burn lesions greater than 30% of body surface, Simplified Acute Physiology Score II of 70 or greater [18], decision to withhold life-sustaining treatment, person deprived of liberty or subject to legal protection measure.
Protocol
Arm selection group
After screening all ARDS patients, we performed an arterial blood gas analysis at a PEEP of 10 cmH2O with the same FiO2 as recorded at screening. If PaO2/FiO2 was less than 100, patients were included in the severe ARDS group. If PaO2/FiO2 was greater than 100, a second arterial blood gas was performed 30 min later at a PEEP of 5 cmH2O. If PaO2/FiO2 was less than 150, patients were included in the moderate ARDS group. If PaO2/FiO2 was greater than 150, the patients were not included in the study and reassessed later.
Mechanical ventilation
All included patients were ventilated according to the original ARDSnet protocol [1]. Briefly, patients were ventilated in a volume-assist control mode with constant square flow and a tidal volume of 6 mL/kg/IPBW (ideal predicted body weight) using the AVEA ventilator (VIASYS Healthcare, Palm Springs, CA). The goal of oxygenation was to target a peripheral saturation of blood oxygen (SpO2) measured by pulse oximetry between 88 and 95% or a PaO2 of 55–80 mmHg measured by arterial blood gas analysis. To achieve this goal, FiO2 and the PEEP were adjusted as in the ARMA and the ACURASYS studies [1, 11]. Respiratory rate was adjusted to ensure arterial pH between 7.20 and 7.45.
Oesophageal and transpulmonary pressure measurements
A specific nasogastric feeding probe (SmartCathG®, VIASYS Healthcare, Palm Springs, CA, USA) equipped with an oesophageal balloon was inserted after in vitro automatized test for leak search and compliance measurement. Then the balloon was filled with a volume of air between 0.5 and 2 mL (as recommended by the manufacturer). Every 30 min, the ventilator evacuates and refills the balloon to maintain measurement accuracy. The correct positioning in the lower third of the oesophagus was confirmed by the presence of cardiac artefacts, the changes in transpulmonary pressure during tidal ventilation and the parallelism of airway and oesophageal curves after a brief chest compression manoeuvre during an airway occlusion test [19]. Finally, a chest X-ray excluded the misplacement of the probe into the airway. Oesophageal pressures were recorded and monitored by the integrated system, a CP-100 pulmonary monitor (Bicore monitoring system Inc ®, Irvine, CA, USA). An end-inspiratory occlusion hold of 2 s allowed the measurement of respectively Pplat and inspiratory Pes (Pesinsp), whereas an end-expiratory occlusion hold of 5 s allowed the measurement of respectively total PEEP (PEEPtot) and expiratory Pes (Pesexp).
The following measurements and calculations were performed at each time point of the study, namely at baseline (without NMBA), 1 h after (H+1) and every 12 h until 48 h (H+12, H+24, H+36, H+48):
Management of sedation
During the 48 h of the study, the Ramsay sedation scale was used to adapt sedative requirements. The scale assigns the conscious state a score of 1 (anxious, agitated, or restless) to 6 (no response on glabellar tap). We used continuous infusion of midazolam and sufentanil to achieve a Ramsay score of 6 throughout the study. If this goal was not achieved, a continuous infusion of ketamine was added.
Neuromuscular blockers administration
Patients with moderate ARDS were randomised according to a computer-generated random-number table stratified by centre and prepared by statisticians to assign patients in blocks of 4 to receive or not a 48-h infusion of cisatracurium besylate. Patients with severe ARDS also received an open-label 48-h infusion of cisatracurium besylate. Cisatracurium besylate was given using a 3-mL rapid intravenous infusion of 15 mg followed by a continuous infusion of 37.5 mg/h. Peripheral nerve stimulation was performed thereafter and a supplementary bolus of cisatracurium besylate was administered if the patient had a response of at least one by the train of four checking.
Data collection
We performed comparisons between two groups of patients with moderate ARDS, the control group and the NMBA group. The results of the moderate ARDS group receiving NMBA were compared to those of the severe ARDS group (receiving NMBA according to the ethics committee statement) in order to verify if our conclusions were generalizable to all ARDS patients with a PaO2/FiO2 less than 150. We collected data on demographic characteristics, severity scores (Simplified Acute Physiologic Score II, Sepsis-related Organ Failure Assessment, McCabe score) [18, 20, 21], risk factors for ARDS, septic shock associated, lactate levels at baseline, and clinical outcomes (the number of days outside the ICU between day 1 and day 28, the number of days without mechanical ventilation between day 1 and day 28 and ICU mortality rate) for each group. We also reported cumulative doses of cisatracurium besylate in the NMBA group.
Statistical analysis
Assuming a reduction of 3 cmH2O of ∆P L at 48 h in the moderate ARDS group receiving NMBA and on the basis of a previously unpublished cohort of ten patients with moderate ARDS receiving cisatracurium besylate and presenting a ∆P L of 8 ± 2.5 cmH2O, the sample size calculation was 11 per group for a 80% statistical power and a two-sided alpha value of 0.05. Assuming technical problems to register a good signal of oesophageal pressure during the 48 h of the protocol, we planned to include 24 patients. The severe ARDS group of patients (all receiving NMBA) was used as comparator to the moderate ARDS group receiving NMBA. We planned to include these patients in the analysis only if there was no difference regarding the assessed parameters. Quantitative variables were expressed as the median and interquartile range (25th and 75th percentiles), and comparisons between groups were performed using the Mann–Whitney U test. Qualitative variables were expressed as the absolute value and percentage and compared using the Fisher exact test. Repeated-measures analysis of variance with Friedman test was used to evaluate the effect of time and group on respiratory mechanics and PaO2/FiO2 ratio. Averages of the respiratory mechanics parameters within each patient were expressed as mean ± standard deviation. After testing the normality of the distribution, comparisons were performed with Student’s test. A p value less than 0.05 was retained as significant. All statistics and figures were performed with the SPSS 20.0 package (SPSS, Chicago, IL, USA).
Results
During a 24-month period, we screened 93 patients with moderate to severe ARDS (Fig. 1). Among them, 61 had exclusion criteria. We initially planned to include 40 patients but the lack of measurement problems of oesophageal pressure and the reduced number of patients in the severe ARDS arm not receiving NMBA at the time of screening allowed us to enrol fewer subjects (i.e. 30). Among them, 24 met moderate ARDS criteria and were randomised into the NMBA group (n = 13) and the control group (n = 11). Results of the randomised study (n = 24 moderate ARDS patients) are provided in Figs. 2, 3 and S3. Results of the open study (n = 6, severe ARDS patients receiving NMBA per protocol) are provided in Figure S1 and pooled data of the entire cohort of 30 patients in Figure S2.
At inclusion median PaO2/FiO2 ratio was 140 (127–146) mmHg with a median PEEP of 10 (6–10) cmH2O in the moderate ARDS group. After optimization of sedation and adequate PEEP level according to the ARMA study, PaO2/FiO2 of the entire moderate ARDS group increased to 152 (127–184) mmHg at baseline. Table 1 compares the demographic data, severity scores, risk factors for ARDS, baseline respiratory mechanics and ICU outcomes between the control and NMBA groups. Both groups were comparable except for slightly higher lactate level at inclusion in the NMBA group.
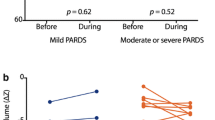
Changes of respiratory mechanics and oxygenation over time are illustrated by Fig. 2. A significant improvement in oxygenation was observed in the moderate ARDS NMBA group during the 48 h of the study, whereas we did not find any improvement of oxygenation in the moderate ARDS control group. Pplat decreased in the moderate ARDS NMBA group but not in the control group. At 24 h, Pplat was 20 IQR (17; 21) cmH2O in the moderate ARDS NMBA group and 22 IQR (19; 26) in the moderate ARDS control group, p = 0.05. We did not find any significant effect of time and group for ∆P values during the study. Most of the patients had low ∆P (the 75th percentile was below the threshold of 15 cmH2O). Concerning the severe ARDS group with per protocol administration of NMBA, we observed an increase in PaO2/FiO2 associated with a decrease in Pplat and PEEP (Fig. S1).
Transpulmonary pressures
A representative tracing of airway, oesophageal and transpulmonary pressures is provided in Fig. 4. While baseline P L values were comparable, a higher inspiratory P L in the NMBA group was recorded at 48 h as compared with the control group (p = 0.01) (Fig. 2). Comparisons between the averages within each patient over the study period of the respiratory mechanic variables are provided in Fig. 3. The average of the inspiratory P L measurements was 8.7 ± 3.3 cmH2O in the NMBA group as compared with 5.7 ± 3.6 cmH2O in the control group (p = 0.04). A higher average of expiratory P L measurements of 1.4 ± 2.7 cmH2O was found in the NMBA group as compared with −1.8 ± 3.5 cmH2O in the control group (p = 0.02) (Fig. 3).
Representative tracing of airway pressure (Wave Paw), oesophageal pressure (Wave Pes) and transpulmonary pressure (Wave Ptp) in a patient with moderate ARDS before and after 2 h of NMBA infusion. Notice the abolition of PEEP swing, the decrease in inspiratory Pes and the increase in P L after NMBA infusion
Interestingly, the percentage of recordings with a positive expiratory P L in the NMBA group was 68% as compared with 42% in the control group (p = 0.008) (Fig. S3). We did not find any effect of time and group on ∆P L (Fig. 2).
Comparisons of inspiratory and expiratory P L between the moderate and severe NMBA group (n = 19) and the control moderate ARDS group (n = 11) are provided in Fig. S2. Inspiratory P L was significantly higher at 1 h and at 48 h in the moderate and severe NMBA groups as compared with the moderate ARDS control group.
NMBA requirements
The median cumulative dose of cisatracurium besylate was 1595 mg IQR (1221; 1830) representing a median infusion rate of 33 mg/h IQR (25; 38).
Discussion
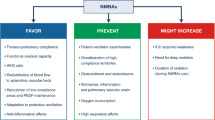
The present study further explored some interesting and favourable effects of continuous cisatracurium besylate infusion during the acute phase of moderate to severe ARDS. We notably demonstrated that the already documented improvement in oxygenation observed with cisatracurium besylate infusion was associated with alterations in transpulmonary pressure regimens. Indeed, we observed higher average of expiratory P L measurements and a greater percentage of positive expiratory P L suggesting less derecruitment during the expiratory phase as well a higher average of inspiratory P L measurements suggesting more recruitment during the inspiratory phase in the cisatracurium besylate group as compared with the control group. The lower oesophageal pressures measured in the NMBA group could reflect the abolition of active expiratory muscles activity but did not seem to be related to the abolition of oesophageal motility according to wave tracings. Cisatracurium besylate infusion was also associated with a reduction of plateau pressure but not with a reduction in driving pressure and transpulmonary driving pressure (∆P L). A delayed and sustained improvement of oxygenation was previously described with the use of continuous cisatracurium besylate infusion [22] and has been discussed elsewhere [12]. The decrease in Pplat has also been previously described but in a longer delays [22].
∆P L, a surrogate of stress applied to the lungs, is related to the strain though the specific elastance of the lungs according the following formula: ∆P L (stress) = E L spec (specific lung elastance) × ∆V/FRC (functional residual capacity) [23]. In our study NMBA infusion did not affect ∆P L which is in agreement with a previous study [24] which found no effect of NMBA either on chest wall elastance or on lung elastance in a heterogeneous group of mechanically ventilated patients.
NMBA infusion was associated with a significantly greater percentage of measured values of positive expiratory P L. Strategies targeting a positive expiratory P L have previously demonstrated their potential clinical utility [25]. This result may, at least partly, explain the favourable effect of NMBA infusion on improvement in oxygenation.
The decrease in plateau pressure during NMBA infusion without any change in ∆P is probably related to the decrease in total PEEP. This latter observation is probably related to lower PEEP levels achieved on the ventilator (according to PEEP/FiO2 table secondary to the improvement in oxygenation) and maybe to a decrease of intrinsic PEEP with NMBA. The higher P L measured in NMBA patients might result from lower oesophageal pressures recorded as a result of the abolition of respiratory muscles activity.
In ARDS patients for whom protective ventilation should be applied, it has been described that different patient–ventilator interactions lead to undesired effects in spite of respirator settings e.g. reverse triggering [26] and breath stacking dyssynchrony [27]. In the latter study, breath stacking dyssynchrony was near-completely eliminated during neuromuscular blockade.
The higher P L exp observed in the NMBA group could be at least partly explained by the fact that in the control group, the relatively high respiratory drive also activates expiratory muscles. Such expiratory muscle activity shifts the diaphragm in the cephalad direction, leading to elevated pleural pressure during expiration and therefore lower P L [28]. Active inspiratory and expiratory efforts could have been assessed by calculating the work of breathing and the pressure–time product of the oesophageal pressure [29, 30]; unfortunately, this was not planned by the protocol and we did not collect these data.
Limitations of the study
First, according the design of the study, we recorded different time points of respiratory mechanics during 48 h. We cannot exclude that a continuous monitoring of P L or a longer period of study would have led to different results. Second, with continuous monitoring and recording of airway and oesophageal pressures, we could have determined which patient–ventilator interactions could be responsible for the differences of P L observed. Third, we used a range of volume to inflate the oesophageal balloon automatized by the ventilator and recommended by the manufacturer. A recent study of Mojoli et al. [31] recommends using a larger volume to inflate the oesophageal balloon of the SmartcathG® catheter. However, our study predates the publication from Mojoli et al. and comparisons between groups are still valid.
And finally, we cannot generalize the results to the more severe patients because we did not randomise those patients. However, the time course of oxygenation and respiratory mechanics in the severe ARDS group are very similar to those of paralysed moderate ARDS patients.
In conclusion, in moderate to severe ARDS patients, we confirm that NMBA infusion during the first 48 h after the onset of ARDS is associated with improvement in oxygenation and we found potent favourable effects on transpulmonary pressures. A further study with continuous recordings of respiratory mechanics including transpulmonary pressures before and after neuromuscular blockers administration could support additional physiological mechanisms.
References
The Acute Respiratory Distress Syndrome Network (2000) Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308. doi:10.1056/NEJM200005043421801
Briel M, Meade M, Mercat A et al (2010) Higher vs lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA 303:865–873. doi:10.1001/jama.2010.218
Guérin C, Reignier J, Richard JC, PROSEVA Study Group et al (2013) Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368:2159–2168. doi:10.1056/NEJMoa1214103
Gao Smith F, Perkins GD, Gates S, BALTI-2 study investigators et al (2012) Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet 379:229–235. doi:10.1016/S0140-6736(11)61623-1
McAuley DF, Laffey JG, O’Kane CM, HARP-2 Investigators, Irish Critical Care Trials Group et al (2014) Simvastatin in the acute respiratory distress syndrome. N Engl J Med 371:1695–1703. doi:10.1056/NEJMoa1403285
National Heart Lung, and Blood Institute ARDS Clinical Trials Network, Truwit JD, Bernard GR, Steingrub J et al (2014) Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med 370:2191–2200. doi:10.1056/NEJMoa1401520
Cornet AD, Groeneveld AB, Hofstra JJ et al (2014) Recombinant human activated protein C in the treatment of acute respiratory distress syndrome: a randomized clinical trial. PLoS One 14:e90983. doi:10.1371/journal.pone.0090983
National Heart Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Matthay MA, Brower RG, Carson S et al (2011) Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med 184:561–568. doi:10.1164/rccm.201012-2090OC
Afshari A, Brok J, Møller AM et al (2010) Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst Rev 7:CD002787. doi:10.1002/14651858.CD002787
Pacheco J, Arnold H, Skrupky L et al (2014) Predictors of outcome in 216 subjects with ARDS treated with inhaled epoprostenol. Respir Care 59:1178–1185
Papazian L, Forel JM, Gacouin A, ACURASYS Study Investigators et al (2010) Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 363:1107–1116. doi:10.1056/NEJMoa1005372
Slutsky AS (2010) Neuromuscular blocking agents in ARDS. N Engl J Med 363:1176–1180. doi:10.1056/NEJMe1007136
Forel JM, Roch A, Marin V et al (2006) Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med 34:2749–2757
Fanelli V, Morita Y, Cappello P et al (2016) Neuromuscular blocking agent cisatracurium attenuates lung injury by inhibition of nicotinic acetylcholine receptor-α1. Anesthesiology 124:132–140. doi:10.1097/ALN.0000000000000907
Jung B, Constantin JM, Rossel N et al (2010) Adaptive support ventilation prevents ventilator-induced diaphragmatic dysfunction in piglet: an in vivo and in vitro study. Anesthesiology 112:1435–1443. doi:10.1097/ALN.0b013e3181d7b036
Yoshida T, Uchiyama A, Matsuura N et al (2012) Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 40:1578–1585
ARDS Definition Task Force, Ranieri VM, Rubenfeld GD, Thompson BT et al (2012) Acute respiratory distress syndrome: the Berlin definition. JAMA 307:2526–2533. doi:10.1001/jama.2012.5669
Le Gall JR, Lemeshow S, Saulnier F (1993) A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA 270:2957–2963
Higgs BD, Behrakis PK, Bevan DR et al (1983) Measurement of pleural pressure with esophageal balloon in anesthetized humans. Anesthesiology 59:340–343
Vincent JL, Moreno R, Takala J et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Mac Cabe WR, Jackson GG (1962) Gram negative bacteremia, etiology and ecology. Arch Intern Med 110:845
Gainnier M, Roch A, Forel JM et al (2004) Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med 32:113–119
Chiumello D, Carlesso E, Cadringher P et al (2008) Lung stress and strain during mechanical ventilation for acute respiratory distress syndrome. Am J Respir Crit Care Med 178:346–355. doi:10.1164/rccm.200710-1589OC
Conti G, Vilardi V, Rocco M et al (1995) Paralysis has no effect on chest wall and respiratory system mechanics of mechanically ventilated, sedated patients. Intensive Care Med 21:808–812
Talmor D, Sarge T, Malhotra A et al (2008) Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med 359:2095–2104. doi:10.1056/NEJMoa0708638
Akoumianaki E, Lyazidi A, Rey N et al (2012) Mechanical ventilation-induced reverse-triggered breaths: a frequently unrecognized form of neuromechanical coupling. Chest 143:927–938
Beitler JR, Sands SA, Loring SH et al (2016) Quantifying unintended exposure to high tidal volumes from breath stacking dyssynchrony in ARDS: the BREATHE criteria. Intensive Care Med 42:1427–1436
Hraiech S, Yoshida T, Papazian L (2015) Balancing neuromuscular blockade versus preserved muscle activity. Curr Opin Crit Care 21:26–33. doi:10.1097/MCC.0000000000000175
Mauri T, Yoshida T, Bellani G et al (2016) Esophageal and transpulmonary pressure in the clinical setting: meaning, usefulness and perspectives. Intensive Care Med 42:1360–1373. doi:10.1007/s00134-016-4400-x
Cabello B, Mancebo J (2006) Work of breathing. Intensive Care Med 32:1311–1314
Mojoli F, Chiumello D, Pozzi M et al (2015) Esophageal pressure measurements under different conditions of intrathoracic pressure. An in vitro study of second generation balloon catheters. Minerva Anestesiol 81:855–864
Acknowledgements
The authors would like to thank all of the nursing team for the excellent care provided to the patients during this study. We thanks Dr. Elizabeth Jouve from APHM, Hôpital La Timone, CIC-UPCET, Pharmacologie Clinique et Evaluations Thérapeutiques, for their methodological councils for conception of the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors state that no significant conflicts of interest exist with regard to any companies or organizations whose products or services may be discussed in this article.
Funding
This project was supported by Assistance Publique-Hôpitaux de Marseille (AO 2011-005720).
Additional information
Take-home message: Neuromuscular blockers form part of the treatment of early-phase moderate to severe acute respiratory distress syndrome.
Trial registration NCT01573715.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guervilly, C., Bisbal, M., Forel, J.M. et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med 43, 408–418 (2017). https://doi.org/10.1007/s00134-016-4653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4653-4