Abstract
Purpose
To review the use of extracorporeal membrane oxygenation (ECMO) in severe paediatric pneumonia and evaluate factors that may affect efficacy of this treatment.
Methods
Retrospective study of the ECMO database of a tertiary paediatric intensive care unit and chart review of all patients who were managed with ECMO during their treatment for severe pneumonia over a 23-year period. The main outcome measures were survival to hospital discharge, and ICU and hospital length of stay. We compared the groups of culture-positive versus culture-negative pneumonia, venoarterial (VA) versus venovenous (VV) ECMO, community- versus hospital-acquired cases, and cases before and after 2005.
Results
Fifty patients had 52 cases of pneumonia managed with ECMO. Community-acquired cases were sicker with higher oxygenation index (41.5 ± 20.5 versus 26.8 ± 17.8; p = 0.031) and higher inotrope score [20 (5–37.5) versus 7.5 (0–18.8); p = 0.07]. Use of VA compared with VV ECMO was associated with higher inotrope scores [20 (10–50) versus 5 (0–20); p = 0.012]. There was a trend towards improved survival in the VV ECMO group (82.4 versus 62.9 %; p = 0.15). Since 2005, patients have been older [4.7 (1–8) versus 1.25 (0.15–2.8) years; p = 0.008] and survival has improved (88.2 versus 60.0 %; p = 0.039).
Conclusions
Survival in children with pneumonia requiring ECMO has improved over time and is now 90 % in the modern era. Risk factors for death include performing a circuit change [odds ratio (OR) 5.0; 95 % confidence interval (CI) 1.02–24.41; p = 0.047] and use of continuous renal replacement therapy (OR 4.2; 95 % CI 1.13–15.59; p = 0.032).
Similar content being viewed by others
Introduction
Pneumonia is the leading cause of death in children worldwide [1, 2], killing an estimated 1.6 million children every year, more than acquired immunodeficiency syndrome, malaria and tuberculosis combined [3]. The incidence of pneumonia is highest in those under 5 years [4].
Pneumonia is a common cause of admission to paediatric intensive care units (PICU). Severe pneumonia commonly requires endotracheal intubation and mechanical ventilation to facilitate adequate oxygenation and carbon dioxide clearance. High-pressure mechanical ventilation for long periods can exacerbate lung injury, leading to chronic lung disease in some patients [5]. For those patients with refractory circulatory or respiratory failure from pneumonia, specialised centres can provide extracorporeal membrane oxygenation (ECMO), where the function of the heart and/or lungs can be supported or supplanted for short periods of time (days or weeks).
The aims of this study are to review the use of ECMO in severe paediatric pneumonia and assess its effect on survival and recovery times in ICU and hospital. We also evaluated factors that influenced patient outcomes, and whether outcomes have changed over the 23-year period that our institution has used ECMO in cases of refractory pneumonia.
Methods
We reviewed the ECMO database of the PICU at the Royal Children’s Hospital, Melbourne, Australia for all children who had received ECMO as part of their management for pneumonia from April 1988 to March 2011. Pneumonia was defined using World Health Organization criteria [3], allowing that proven infection in any part of the lower respiratory tract constituted pneumonia [1]. All patient information is entered prospectively into the database, which is approved by the hospital Ethics Review Board. Permission from the Ethics Review Board was granted to review this information. We included all patients who were supported with venovenous (VV) or venoarterial (VA) ECMO for primary respiratory failure or acute cardiovascular deterioration during treatment for infective respiratory failure. These were then cross-referenced with hospital pathology and radiology services, as well as individual patient records, to confirm both microbiological and radiological evidence of pneumonia at time of commencing ECMO. All patients included met the definition of pneumonia. The primary outcome measure was survival to hospital discharge. All patients were managed empirically upon admission using specific hospital guidelines regarding investigation and appropriate antibiotics [6].
Our institutional approach to VA and VV ECMO has been presented elsewhere [7–9]. Our unit has an established system with which to implement ECMO when needed. If a patient has refractory hypoxia or worsening multi-organ disease despite appropriate ventilatory and pharmacologic strategies, ECMO can be instituted at any time. Cannulation is performed by a cardiac surgeon under general anaesthesia in the paediatric intensive care unit. After the year 2000 we have exclusively used constrained vortex pumps (Rotaflow; Maquet, Hirrlingen, Germany) and hollow-fibre oxygenators (Quadrox; Maquet, Hirrlingen, Germany and HiLite; Medos, Stolberg, Germany). Prior to 2000, we used Biomedicus pumps (Medtronic Biomedicus, Eden Prairie, MN, USA) and Avecor (Medtronic, Minneapolis, MN, USA) or Scimed (SciMed, Minneapolis, MN, USA) oxygenators. Two primed ECMO circuits are always kept on standby for immediate use. Our usual practice for VA ECMO is to target flows of 150 mL/kg/min in children weighing <10 kg and 2.4 L/min/m2 in children weighing >10 kg. Venovenous flow is targeted to SaO2 >80 % in those without congenital heart disease [10]. Once stable on ECMO, inotropes are weaned off as tolerated and ventilatory settings are reduced to ‘rest’ settings in order to allow cardiac and pulmonary recovery, e.g. positive end-expiratory pressure (PEEP) 5–12 cmH2O, peak inspiratory pressure <25 cmH2O, rate 8–10 breaths per minute. Monitoring of both plasma-free haemoglobin (measured daily) and continuous monitoring of pump inlet pressures (>−20 mmHg, measured at the first division of the cannula and tubing) are used to identify haemolysis and adequacy of inflow. Anticoagulation is maintained by continuous intravenous unfractionated heparin targeting an activated clotting time (MAX-ACT; Helena Laboratories Corporation, Beaumont, TX, USA) of 160–180 s. If there is excessive bleeding, this target is reduced to 140–160 s. The platelet count is maintained >80–100 × 109/L, and if ongoing bleeding occurs, tranexamic acid is considered (10–15 mg/kg every 8 h).
We measured and recorded demographic data, respiratory parameters and ventilator settings, and cardiovascular parameters and interventions such as vasoactive medications. We measured these parameters before, during and after ECMO, and collected data on durations of treatment and time in PICU and the hospital.
Continuous variables were assessed for normality and expressed as mean ± standard deviation (SD), and categorical variables were expressed as counts and proportions. Ordinal variables were presented as medians (inter-quartile ranges). A comparison between groups was performed using the Mann–Whitney U test for nonparametric continuous variables, Student t test for parametric continuous variables and chi-square or Fisher’s exact test as appropriate for categorical variables. Logistic regression analysis was used to assess risk factors for hospital mortality, with results reported as odds ratios (ORs) and 95 % confidence interval (CI’s). A two-sided p value of 0.05 was considered statistically significant. All analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC, USA).
Results
From April 1988 to March 2011 there were 467 episodes of ECMO at our institution. Of these, 52 (11.1 %) had a primary diagnosis of pneumonia when commencing ECMO, of whom 35 (67.3 %) had both radiological and microbiological evidence of pneumonia. Seventeen (32.7 %) patients had clinical and radiological but not microbiological evidence of pneumonia. This latter group was classified as culture-negative pneumonia for the purpose of this study. There were no statistically significant differences between these two groups, other than more frequent use of inhaled nitric oxide in the culture-negative group (52 versus 25 %; p = 0.05) and trends in the culture-positive group towards increased use of VA ECMO (74 versus 52 %; p = 0.12) and longer hospital stay [30 (16–46) versus 18 (14–28) days; p = 0.14]. Of the 35 culture-positive cases, 20 (57.1 %) were bacterial, 10 (28.6 %) were viral, 2 (5.7 %) were fungal and 3 (8.6 %) were mixed viral and bacterial cultures. The majority of these (68.6 %) were cultures from bronchoalveolar lavage.
Of the 52 patients included, median age and weight were 1.75 (0.3–5.4) years and 12.0 (5.0–19.0) kg, respectively. Fifteen (28.8 %) patients received high-frequency oscillatory ventilation (HFOV) prior to ECMO, and 18 (34.6 %) received inhaled nitric oxide (iNO). Thirty-two (61.5 %) patients received neither of these treatments, and 14 (26.9 %) received both. Those who received either therapy had a greater likelihood to subsequently receive VV than VA ECMO (iNO 64 versus 20 %, p = 0.001; HFOV 58 versus 17 %, p = 0.002).
Pre-ECMO ventilatory parameters across sub-groups showed no significant difference. Overall, the mean airway pressure was 22.5 (18.3–27.0) cmH2O, with positive end-expiratory pressure of 10.0 (6.0–12.0) cmH2O. The median pH was 7.31 (7.18–7.42), and the median PaCO2 was 50.0 (39.5–58.5). The median PaO2/FiO2 was 63.1 (47.0–96.0), and the mean oxygenation index (OI) was 37.9 ± 20.7. Twelve (23.1 %) patients had OI <20, 13 (25.0 %) had OI 20–39 and 24 (46.2 %) had OI ≥40. The OI was unknown in three (5.8 %) patients who were hand-ventilated onto ECMO. Thirty-five (67.3 %) patients had documented multi-organ dysfunction syndrome, with 16 (30.7 %) who had oligoanuria, 9 (17.3 %) who had disseminated intravascular coagulation and 4 (7.7 %) who had seizures. Forty-one (78.8 %) patients received an infusion of at least one inotrope, with a median inotrope score [11] of 15 (5–30). Two patients (3.8 %) required adrenaline boluses. Nine (17.3 %) patients became bradycardic or asystolic, requiring cardiac massage before commencing ECMO.
The median time lapse between admission to PICU and initiation of ECMO was 20.6 h (2.43–48.8 h) overall, and in patients pre- and post-2005 was 2.1 (0–32) and 24 (4.7–49.5) h, respectively. Univariate analysis examining the relationship between time of ECMO initiation and death in the two eras yielded OR of 0.998 (CI 0.992–1.004). Thirty-five (67.3 %) were cannulated onto VA ECMO. Seventeen (32.7 %) patients were cannulated onto VV ECMO (Table 1).
Overall, patients had a median ECMO duration of 176.6 (91.7–347.9) h. A comparison between the survivors and non-survivors is presented in Table 2. Thirty-seven (71.2 %) patients survived to decannulation from ECMO, and 1 patient later died in hospital, leaving 36 (69.2 %) patients surviving to hospital discharge. Sixteen (30.8 %) patients developed irreversible organ failure and had limitations of life-sustaining therapies instituted. In the survivors, median duration of PICU stay was 16.0 (10.8–26) days. Survivors were more likely if treated after 2005 (88.2 versus 60.0 %; p = 0.039), and had longer non-ECMO ventilation hours [187 (77.6–415.9) versus 54.2 (24–195.5) h; p = 0.009] and longer stays in hospital [31.0 (20.2–46.3) versus 14.5 (10.3–17.5) days; p = 0.0009]. One (1.9 %) patient required three ECMO runs during their overall illness, in the end surviving to hospital discharge. Analysis showed that risk factors for death included performing a circuit change (OR 5.0; 95 % CI 1.02–24.41; p = 0.047) and use of continuous renal replacement therapy (OR 4.2; 95 % CI 1.13–15.59; p = 0.032).
Complications while on ECMO were common, although these include all complications as reported to the Extracorporeal Life Support Organization (ELSO) database and the majority of these were trivial. Five (9.6 %) patients had no complications. Forty-three (82.7 %) had at least one patient-related complication, and 39 (75 %) patients had at least one mechanical complication. Of the significant mechanical complications, 6 (11.5 %) patients had oxygenator failure, 5 (9.6 %) had pump failure, 5 (9.6 %) required cannula repositioning and 25 (48.1 %) had clots visible in the circuit. The latter was more common in VV than VA ECMO (76 versus 31 %; p = 0.002). Ten (19.2 %) patients required a circuit change, which was associated with higher mortality (31 % of the non-survivors versus 8 % of the survivors had circuit changes, p = 0.035). Of the significant patient-related complications, two (3.8 %) had seizures, three (5.8 %) had a cerebral infarction, and two (3.8 %) were certified brain dead. Six (11.5 %) patients had significant haemolysis (plasma Hb >1.0 g/dL) and 13 (25.0 %) had bleeding requiring re-exploration. Two (3.8 %) patients had a pneumothorax requiring treatment, and three (5.8 %) had a pulmonary haemorrhage. Twenty (38.5 %) patients became bacteraemic after commencing ECMO, which was more commonly associated with VV than VA ECMO (64 versus 25 %; p = 0.007).
Forty (76.9 %) patients had community-acquired pneumonia (CAP), and 12 (23.1 %) were hospital-acquired cases (Table 3). Patients with CAP had higher inotrope scores [20 (5–37.5) versus 7.5 (0–18.8); p = 0.07] and OI (41.5 ± 20.5 versus 26.8 ± 17.8; p = 0.031). They were admitted to PICU and intubated for shorter durations prior to ECMO [15.2 (0.7–28) versus 56.7 (20.3–151.3) h; p = 0.005; and 30.4 (17.3–91.6) versus 101.8 (51–378.9) h; p = 0.043, respectively] than the hospital-acquired group, but trended towards a shorter hospital stay [20.7 (14.4–35.1) versus 40.1 (19–99.4) h; p = 0.09].
Table 4 compares the groups pre- and post-2005. Patients after 2005 were significantly older [4.7 (1–8) versus 1.25 (0.15–2.8) years; p = 0.008] and more likely to survive (88.2 versus 60.0 %; p = 0.039). Both groups had equivalent levels of ventilator support and inotrope scores, although the OI was non-significantly lower in the post-2005 group (32.8 ± 23.1 versus 40.1 ± 19.5; p = 0.26). The need for cardiac compression pre-ECMO was significantly more frequent after 2005 (35 versus 8 %; p = 0.017). Inhaled nitric oxide was used more frequently after 2005 (52 versus 25 %; p = 0.05). Incidence of bacteraemia after commencing ECMO was more common after 2005 (64 versus 25 %; p = 0.007), as was detection of clots within the ECMO circuit (70 versus 34 %; p = 0.014).
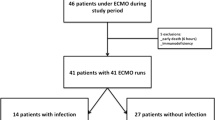
A flowchart of data from a comparable timeline to the post-2005 cohort, taken from another retrospective database review, is shown in Fig. 1. Our institutional approach for second-tier therapies is to utilise HFOV, aggressive fluid restriction, inhaled nitric oxide, and steroids. We do not routinely use prone positioning or surfactant in children outside the neonatal period. The data for second-tier therapies other than HFOV (e.g. steroids, inhaled nitric oxide and surfactant) in those who were not ultimately supported with ECMO were insufficiently complete to be analysed.
Flowchart of patients with pneumonia in the RCH PICU from 2005 RCH PICU Royal Children’s Hospital Paediatric Intensive Care Unit, HFOV high-frequency oscillatory ventilation, ECMO extracorporeal membrane oxygenation. PIM2 scores of predicted risk of death are provided as medians (interquartile range)
Discussion
The results of our study of a 23-year period show that survival to hospital discharge in children with pneumonia admitted to our PICU and supported with ECMO was 69.2 %. When the analysis was confined to 2005 onward, outcomes were more favourable, with survival rates of 88.2 %. There was no statistically significant difference between the two eras in pre-ECMO ventilatory parameters or arterial blood gases. The improvement in survival in the modern era approaches that of the non-ECMO group, who represent the majority of pneumonia that presents to PICU, although whether this is due to better circuit technology, improved clinical management, more refined patient selection or other unknown factors remains unclear. On the basis of our PIM2 data of the different tiers, we speculate that it is not entirely due to patient selection.
The incidence of ECMO use in severe pneumonia has fluctuated over time. The exclusive use of VA ECMO in the late 1980s fell drastically, as did the use of VV ECMO after a brief rise in frequency in the mid 1990s. At the start of the new century, both began to rise again and are currently equally employed (on average two cases per year of each mode for the past 3 years). A number of factors require consideration when the suitability of ECMO is being assessed for a child with severe pneumonia, in particular the rate of decline and whether other rescue therapies have been tried. Our results show that other adjunctive therapies such as HFOV or inhaled nitric oxide were considered and often provided if cardiovascular stability permitted their use prior to ECMO. These results are supported by other studies [12–14]. If the patient showed severe signs of cardiovascular compromise, VA ECMO was commenced instead of a trial of other rescue therapy. Venovenous ECMO patients were significantly more likely to have tried an adjunctive therapy prior to cannulation, although we did not capture data on rescue therapy used in other centres prior to transfer to our institution. Since September 2003 we have offered a retrieval service that goes to the referring hospital and cannulates the patient onto ECMO prior to transport [15]. Since initiating this service, an increasing number of ECMO transfers have been completed, and the instability in transport and need for urgent VA ECMO on arrival to PICU have lessened.
Another factor to consider when choosing a cannulation strategy is the inotrope score, a sum of the requirement for dobutamine, dopamine, adrenaline and/or noradrenaline [11]. As shown by the median inotrope scores prior to ECMO (VA 20 versus VV 5; p = 0.012), the greater the need for inotropes, the more likely VA ECMO would be selected. As lung disease worsens and increased ventilatory pressures are required, decreased venous return and increased pulmonary vascular resistance from high intrathoracic pressures cause circulatory dysfunction [16]. This may be compounded by sepsis-induced cardiovascular changes.
The majority of patients experienced at least one complication attributed to ECMO, with the median number of complications being 4.5 in survivors and 6 in non-survivors, although most were trivial, e.g. electrolyte disturbances or the need for vasoactive drugs while on support. Other centres have found similar high complication rates [17]. The most serious complications, e.g. brain death, cerebral infarction and seizures, were all seen in the VA ECMO group and may represent the increased risk of systemic thromboembolism and maldistribution of oxygenated blood seen in patients supported on VA ECMO. Two complications seen more commonly in the VV group were nosocomial infection and circuit clots. This may have been related to the increased duration of support seen in the VV group.
Our study has a number of weaknesses. It is a single-centre, retrospective study analysing data over a 23-year period. Changes in practice over this time may make our results harder to interpret, although we tried to address this by separately analysing a patient cohort in the modern era. The lack of prospective analysis makes it difficult to offer conclusive recommendations on cannulation strategies.
Conclusions
Survival in children with pneumonia supported with ECMO in our institute in the modern era is 90 % and is almost comparable to patients just requiring invasive mechanical ventilation. Venoarterial ECMO may be associated with more serious complications and should be reserved for children with profound haemodynamic instability and ventricular dysfunction. Risk factors for poor outcome include the need to change the ECMO circuit and the need for renal replacement therapy.
References
Langley JM, Bradley JS (2005) Defining pneumonia in critically ill infants and children. Pediatr Crit Care Med 6:S9–S13. doi:10.1097/01.PCC.0000161932.73262.D7
Singh V, Aneja S (2011) Pneumonia—management in the developing world. Paediatr Respir Rev 12:52–59. doi:10.1016/j.prrv.2010.09.011
World Health Organisation (2010) Pneumonia Factsheet. Available from: http://www.who.int/mediacentre/factsheets/fs331/en/index.html Accessed 10 May 2011
Prayle A, Atkinson M, Smyth A (2011) Pneumonia in the developed world. Paediatr Respir Rev 12:60–69. doi:10.1016/j.prrv.2010.09.012
Bayrakci B, Josephson C, Fackler J (2007) Oxygenation index for extracorporeal membrane oxygenation: is there predictive significance? J Artif Organs 10:6–9. doi:10.1007/s10047-006-0359-7
Young S, South M (2007) Clinical practice guideline for pneumonia. Available from: http://www.rch.org.au/clinicalguide/cpg.cfm?doc_id=5285 Accessed 31 May 2011
MacLaren G, Butt W, Best D, Donath S (2011) Central extracorporeal membrane oxygenation for refractory pediatric septic shock. Pediatr Crit Care Med 12:133–136. doi:10.1097/PCC.0b013e3181e2a4a1
MacLaren G, Butt W, Best D, Donath S, Taylor A (2007) Extracorporeal membrane oxygenation for refractory septic shock in children: one institution’s experience. Pediatr Crit Care Med 8:447–451. doi:10.1097/01.PCC.0000282155.25974.8F
Macintosh I, Butt WW, Robertson CF, Best D, Shekerdemian LS (2005) Extending the limits of extracorporeal membrane oxygenation: lung rest for a child with non-specific interstitial pneumonia. Intensive Care Med 31:993–996. doi:10.1007/s00134-005-2620-6
Extracorporeal Life Support Organisation (2009) ELSO Guidelines Version1.1 1–24. Available from: http://www.elso.med.umich.edu/Guidelines.html Accessed 10 May 2011
Wernovsky G, Wypij D, Jonas RA, Mayer J, Hanley F, Hickey P, Walsh A, Chang A, Castaneda A, Newburger J, Wessel D (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants: a comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235. doi:10.1161/01.CIR.92.8.2226
Mehta NM, Turner D, Walsh B, Zurakowski D, Betit P, Wilson J, Arnold J (2010) Factors associated with survival in pediatric extracorporeal membrane oxygenation–a single-center experience. J Pediatr Surg 45:1995–2003. doi:10.1016/j.jpedsurg.2010.05.028
Norfolk SG, Hollingsworth CL, Wolfe CR, Govert J, Que L, Cheifetz I, Hollingsworth J (2010) Rescue therapy in adult and pediatric patients with pH1N1 influenza infection: a tertiary center intensive care unit experience from April to October 2009. Crit Care Med 38:2103–2107. doi:10.1097/CCM.0b013e3181f268f1
Westrope C, Roberts N, Nichani S, Hunt C, Peek G, Firmin R (2004) Experience with mobile inhaled nitric oxide during transport of neonates and children with respiratory insufficiency to an extracorporeal membrane oxygenation center. Pediatr Crit Care Med 5:542–546. doi:10.1097/01.PCC.0000137338.27059.C7
Perez A, Butt WW, Millar KJ, Best D, Thiruchelvam T, Cochrane AD, Bennett M, Shekerdemian LS (2008) Long-distance transport of critically ill children on extracorporeal life support in Australia. Crit Care Resusc 10:30–34
Shekerdemian LS, Bohn D (1999) Cardiovascular effects of mechanical ventilation. Arch Dis Child 80:475–480
Adolph V, Heaton J, Steiner R, Bonis S, Falterman K, Arensman R (1991) Extracorporeal membrane oxygenation for nonneonatal respiratory failure. J Pediatr Surg 26:326–332. doi:10.1016/0022-3468(91)90511-Q
Acknowledgments
We thank Michelle Goldsworthy, Carmel Delzoppo and Mitch Shein for their help in data extraction, and the cardiac surgeons (Christian Brizard, Yves D’Udekem and Igor Konstantinov) and perfusionists who contribute their expertise in the care of these patients.
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Smalley, N., MacLaren, G., Best, D. et al. Outcomes in children with refractory pneumonia supported with extracorporeal membrane oxygenation. Intensive Care Med 38, 1001–1007 (2012). https://doi.org/10.1007/s00134-012-2581-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-012-2581-5