Abstract
Background
Ventilator-induced lung injury (VILI) occurs in part by increased vascular permeability and impaired alveolar fluid clearance. Phosphoinositide 3-kinase gamma (PI3Kγ) is activated by mechanical stress, induces nitric oxide (NO) production, and participates in cyclic adenosine monophosphate (cAMP) hydrolysis, each of which contributes to alveolar edema. We hypothesized that lungs lacking PI3Kγ or treated with PI3Kγ inhibitors would be protected from ventilation-induced alveolar edema and lung injury.
Methods
Using an isolated and perfused lung model, wild-type (WT) and PI3Kγ-knockout (KO) mice underwent negative-pressure cycled ventilation at either −25 cmH2O and 0 cmH2O positive end-expiratory pressure (PEEP) (HIGH STRESS) or −10 cmH2O and −3 cmH2O PEEP (LOW STRESS).
Results
Compared with WT, PI3Kγ-knockout mice lungs were partially protected from VILI-induced derangement of respiratory mechanics (lung elastance) and edema formation [bronchoalveolar lavage (BAL) protein concentration, wet/dry ratio, and lung histology]. In PI3Kγ-knockout mice, VILI induced significantly less phosphorylation of protein kinase B (Akt), endothelial nitric oxide synthase (eNOS), production of nitrate and nitrotyrosine, as well as hydrolysis of cAMP, compared with wild-type animals. PI3Kγ wild-type lungs treated with AS605240, an inhibitor of PI3Kγ kinase activity, in combination with enoximone, an inhibitor of phosphodiesterase-3 (PDE3)-induced cAMP hydrolysis, were protected from VILI at levels comparable to knockout lungs.
Conclusions
Phosphoinositide 3-kinase gamma in resident lung cells mediates part of the alveolar edema induced by high-stress ventilation. This injury is mediated via altered Akt, eNOS, NO, and/or cAMP signaling. Anti-PI3Kγ therapy aimed at resident lung cells represents a potential pharmacologic target to mitigate VILI.
Similar content being viewed by others
Introduction
Mechanical ventilation is a life-saving therapy but can initiate or exacerbate lung injury [1–4]. This ventilation-induced lung injury (VILI) results, in part, in increased vascular permeability and impaired alveolar fluid clearance, resulting in the clinical manifestation of alveolar edema [5–7].
Pulmonary mechanical stretch enhances nitric oxide (NO) production [8, 9], which can contribute to the pathogenesis of VILI by increasing alveolar and vascular permeability [10–12]. This mechanism is thought to involve peroxynitrite, which is a potent oxidant produced by the interaction of NO and oxygen radicals [13]. Nitrotyrosine, a marker of peroxynitrite damage, is increased in patients with acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) [13–15]. In addition to permeability alterations, reactive nitrogen species can impair cyclic adenosine monophosphate (cAMP)-dependent alveolar fluid clearance (AFC) [11]. The intracellular level of cAMP is a critical modulator of endothelial permeability, and strategies that increase cAMP can reverse alterations in lung permeability [16, 17].
The phosphoinositide 3-kinase (PI3K) family is found largely in neutrophils, but is also expressed in structural cells of various organs including the lung [18], where they contribute to cyclic, stretch-activated nitric oxide production in endothelial cells [19]. Since the PI3Kγ isoform can act independently of its kinase activity to modulate cAMP concentrations [20, 21], it may play an essential role in the formation of edema during VILI. Recently, our group demonstrated a role for PI3Kγ in VILI [22]; however, that study did not examine the effect on pulmonary edema formation, nor identify whether the cells mediating VILI were the circulating neutrophils or the residential cells of the lung, which could be specifically targeted by anti-PI3Kγ therapies. Therefore, use of inhibitors towards the kinase-dependent and/or kinase-independent functions of PI3Kγ may each induce protection against the detrimental effects of VILI.
The aim of this study is to investigate the role of pulmonary-derived PI3Kγ in the development of ventilator-induced lung injury and edema. We hypothesized that isolated and perfused lungs lacking PI3Kγ would be protected from VILI and edema formation, through the alteration of NO signaling and cAMP lung content. Moreover, we hypothesized that this protection would result from a combination of the blockade of both the kinase-dependent and kinase-independent effects.
Materials and methods
Animals, surgery, and ventilation protocol
Experiments were conducted on 129/Sv adult male PI3Kγ wild-type (PI3Kγ+/+) and knockout (PI3Kγ−/−) mice (25–30 g). PI3Kγ wild-type and knockout lungs were isolated, perfused, and ventilated as previously described [23]. After ascertaining pulmonary perfusion, the mode of ventilation was changed to negative pressure to minimize hydrostatic edema formation [23]. After stabilization (60 min), lungs were randomized to two ventilation strategies: LOW STRESS, with end-inspiratory pressure (EIP) = −10 cmH2O and end-expiratory pressure (EEP) = −3 cmH2O; and HIGH STRESS, with EIP = −25 cmH2O and EEP = 0 cmH2O. Ventilation was stopped after either 60 or 180 min. Animals used as negative controls (n = 8, NO STRESS group) were anesthetized and exsanguinated, lung vasculature was flushed, and the hearts and lungs were dissected en bloc without mechanical ventilation or perfusion.
Lung function and morphology
Airway opening pressure (Pao), pressure inside the chamber, and tidal volume (Vt) as the integral of the flow, were measured [24]. When ventilation was stopped after 1 h, lung tissue was isolated, snap-frozen, and stored at –80°C. When ventilation was stopped after 3 h, the right lung was excised, snap-frozen, and stored at –80°C. The left lung was then inflated and fixed with 4% paraformaldehyde (Sigma–Aldrich, Milwaukee, WI, USA) [25]. Lung sections (3 μm) were stained with hematoxylin and eosin, and nine random fields of view were analyzed by two pathologists blinded to experimental groups. For histopathological evaluation of lung damage, two separate scores relating to edema and inflammatory cell infiltration were calculated. Interstitial edema was scored as: 0, absent; 1, focally present; 2, diffusely present. Alveolar edema was scored as: 0, absent; 1, mild, focal; 2, mild, diffuse; 3, severe, focal; 4, severe, diffuse. Total lung edema was scored by adding the interstitial and alveolar edema scores. Endoalveolar macrophage quantification (alveolar macrophage score) was scored as: 0, absence; 1, mild infiltration; 2, moderate infiltration; 3, severe infiltration.
BAL protein concentration
The right lung was lavaged four times with 0.5 mL phosphate-buffered saline (PBS). Total protein concentration was measured by Micro BCA protein assay (Pierce, Rockford, IL), as per the manufacturer’s instructions.
Lung weight-to-dry (W/D) ratio
After 3 h of ventilation, the left lung was tied at the left hilum, weighed (wet weight), desiccated at 80°C for 1 week, then reweighed (dry weight).
Lung protein expression
Expression of phosphorylated and/or total protein kinase B (Akt), endothelial nitric oxide synthase (eNOS), and inducible nitric oxide synthase (iNOS) was measured by Western blot using monoclonal antibodies (Cell Signaling Technology; BD Biosciences). Densitometry was analyzed using Quantity One 4.6.1 software (BioRad, CA).
Perfusate analysis of nitrite/nitrate and cytokines
Perfusate samples were collected every 30 min throughout ventilation from the left atrium cannula. Nitrite/nitrate generation in lung perfusate samples was measured by Griess reagent (Sigma). Perfusate levels of interleukin-6 (IL6) and macrophage inflammatory protein-2 (MIP2) (R&D Systems, Minneapolis, MN) were assessed by enzyme-linked immunosorbent assay (ELISA).
Immunohistochemistry of pAkt, peNOS, and nitrotyrosine
Paraffin-fixed lung sections were incubated with primary antibodies against pAkt (Cell Signaling Technology), peNOS (BD Transduction Laboratories, USA) or nitrotyrosine (clone HM11; Santa Cruz Biotechnology). Immunohistochemical quantitation of nitrotyrosine accumulation in lung tissue was evaluated in the alveolar wall, endoalveolar acellular material, and macrophages and was scored as: 0, absent; 1, 1–5% of the evaluated structure; 2, 5–10% of the evaluated structure; 3, >10% of the evaluated structure.
Determination of cAMP content in lungs
Lung tissue cAMP content was determined by radiolabeled competitive protein binding assay (Cyclic AMP 3H Biotrak Assay System; GE Healthcare Life Science, Buckinghamshire, UK), as per the manufacturer’s instructions.
Determination of myeloperoxidase in lungs
Myeloperoxidase was measured in lung homogenate using an enzyme-linked immunosorbent assay kit, according to the manufacturer’s instructions (Hycult Biotechnology, Uden, The Netherlands). Lungs from septic mice [induced by cecal ligation and perforation (CLP)] [26] were used as a positive control.
Treatment with the PI3Kγ inhibitor AS605240 and enoximone
In wild-type animals, AS605240, a PI3Kγ inhibitor [27], was administered subcutaneously (10 mg/kg) 16 h before ventilation and was added to the perfusate (0.1 mg/kg/min) during ventilation. Enoximone (Perfan; INCA-Pharm), a phosphodiesterase-3 (PDE3) inhibitor, was used to mimic the increased cAMP observed in PI3Kγ-knockout mice. In wild-type and knockout animals, enoximone was administered ex vivo into the pulmonary artery (1 mg/kg bolus) and into the perfusate (90 μg/kg/min). Additionally, in wild-type animals, both AS605240 and enoximone were administered. Control animals received the respective drug vehicles. During the 3 h of HIGH STRESS or LOW STRESS ventilation, lung perfusate samples were obtained for nitrite/nitrate determination. Following ventilation, dynamic lung compliance was calculated as: CLUNG = tidal volume/(end inspiratory pressure − end expiratory pressure). The left lung was obtained for cAMP determination and pAkt expression, and BAL was performed on the right lung for total protein determination.
Statistics
Results are presented as mean ± standard error of the mean (SEM). Comparison among groups was performed using the Kruskal–Wallis test, followed by Dunnett multiple-comparison post hoc analysis. A p value <0.05 was considered statistically significant (SPSS Inc., Chicago, IL).
Results
Respiratory mechanics and vascular permeability changes
During the initial 60 min stabilization period prior to randomization, tidal volume (Vt) did not differ among groups. Immediately following randomization, there was a significant increase in Vt in both HIGH STRESS groups (Fig. 1a). However, from 20 min until the end of the experimental period, the Vt of the HIGH STRESS−/− group remained significantly higher than that of the HIGH STRESS+/+ group (p < 0.05; Fig. 1a).
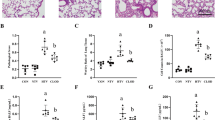
a Changes in tidal volume versus time. Given that we used a fixed transpulmonary pressure throughout the study, decreases in tidal volume over time are a surrogate for decreases in lung compliance. Experimental groups are: LOW STRESS (EIP −10 cmH2O, EEP −3 cmH2O) wild type (+/+) and PI3Kγ knockout (−/−), HIGH STRESS (EIP −25 cmH2O, EEP 0 cmH2O) wild type (+/+) and PI3Kγ knockout (−/−), and NO STRESS (flushed but no ventilation) wild type (+/+) and PI3Kγ knockout (−/−), where n = 8 for all groups. *HIGH STRESS−/− was significantly different from all other groups, starting from 20 min and throughout the experimental period. b and c BAL protein concentrations and W/D weight ratios of ventilated and perfused lungs. *HIGH STRESS+/+ was significantly different from all other groups; °HIGH STRESS−/− was significantly different from both LOW STRESS and NO STRESS groups
HIGH STRESS induced significant increases in BAL protein and W/D ratio compared with controls. Lungs from HIGH STRESS+/+ animals had significantly increased BAL protein concentration and edema (p < 0.05) compared with lungs of HIGH STRESS−/− animals (Fig. 1b, c).
Lung edema and alveolar macrophage scores
In NO STRESS animals there were no observable differences in lung morphology or edema score (Fig. 2a, b). Following HIGH STRESS ventilation, wild-type mice had higher edema scores compared with knockout (p < 0.01), LOW STRESS and NO STRESS (p < 0.001) animals. By contrast, HIGH STRESS knockout lungs developed higher lung edema scores only compared with NO STRESS (p < 0.05).
Histological assessment of lung edema and macrophage infiltration: a representative lung micrographs stained with hematoxylin and eosin (200× magnification); b histological lung edema score: combined assessment of alveolar and interstitial edema formation. *The HIGH STRESS+/+ group was significantly different from all the other groups; °The HIGH STRESS−/− group was different from both NO STRESS groups. c Alveolar macrophage score: an assessment of alveolar macrophages infiltration. *Both HIGH STRESS groups were different from all other groups. Experimental groups and the number of animals are as in Fig. 1
During HIGH STRESS ventilation there was a statistically significant increase in the alveolar macrophages score compared with LOW STRESS and NO STRESS (p < 0.05), but there was no significant difference between genotypes (Fig. 2c).
Intracellular signaling activation
Phosphorylated Akt, eNOS (Fig. 3a, b), and iNOS (ESM Fig. 1) were each not significantly different between LOW STRESS+/+ and LOW STRESS−/−. However, HIGH STRESS+/+ lungs had significantly increased phosphorylated Akt and eNOS compared with HIGH STRESS−/− (p < 0.05), LOW STRESS, and NO STRESS groups (p < 0.05; Fig. 3a, b). The HIGH STRESS−/− group exhibited a significant increase in pAkt and peNOS compared with only NO STRESS (p < 0.05; Fig. 3a, b). The concentration of iNOS was not different between groups (ESM Fig. 1).
Ventilation-induced activation of: a pAkt and b phosphorylated endothelial NOS (peNOS), assessed in lung homogenate by Western blot. Densitometric values are displayed as a phosphorylated/unphosphorylated ratio fold increase. Experimental groups are identical to those described in Fig. 1, and n = 5 for all groups. c Time course of nitrate/nitrite concentration in lung perfusate, with n = 8 for all groups. *HIGH STRESS+/+ was different from all other groups
Immunohistochemistry for pAkt demonstrated that LOW STRESS−/− lungs had less alveolar staining (brown) than LOW STRESS+/+ (ESM Fig. 2). HIGH STRESS+/+ had qualitatively more pAkt staining than LOW STRESS controls, which was nearly completely blocked in the HIGH STRESS−/− group. Immunohistochemistry for peNOS confirmed that eNOS activation in both LOW STRESS groups was minimal (ESM Fig. 3). In the HIGH STRESS+/+ group, alveolar cells were strongly positive for peNOS, while the HIGH STRESS−/− group displayed observably less staining. NO STRESS groups displayed no observable difference in pAkt or peNOS staining (ESM Figs. 2, 3).
Nitrate/nitrite and cytokines release from the lung
The nitrate/nitrite levels in HIGH STRESS−/− were equal to those of LOW STRESS lungs (Fig. 3c). However, in HIGH STRESS+/+ there was a progressive increase in nitrate/nitrite throughout the 180 min of ventilation compared with HIGH STRESS−/− (p < 0.05) and LOW STRESS groups (p < 0.05). Perfusate IL6 and MIP2 concentrations increased over time in both LOW STRESS and HIGH STRESS groups, but increased more rapidly in HIGH STRESS groups (ESM Fig. 4). Cytokine concentrations were not significantly different between knockout and wild-type lungs within each ventilation strategy.
Nitrotyrosine immunohistochemistry
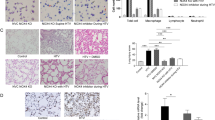
HIGH STRESS+/+ demonstrated the greatest nitrotyrosine staining (Fig. 4a), with high accumulation in the alveolar wall compared with HIGH STRESS−/− (p < 0.05; Fig. 4b). Lung macrophage nitrotyrosine accumulation also tended to increase in HIGH STRESS+/+ compared with HIGH STRESS−/− (Fig. 4c), but did not reach statistical significance.
Lung nitrotyrosine analysis. a Representative immunohistochemical staining of nitrotyrosine; note the highest staining intensities for nitrotyrosine in the alveolar cells in the HIGH STRESS+/+ group. b Lung epithelial cell nitrotyrosine score: an assessment of alveolar epithelial cells that have positive staining. *HIGH STRESS+/+ was significantly different from all other groups. c Histological lung macrophages nitrotyrosine score: an assessment of macrophage positive staining. *HIGH STRESS+/+ was significantly different from LOW STRESS and NO STRESS groups; °HIGH STRESS−/− was significantly different from NO STRESS groups. Experimental groups are the same as those described in Fig. 1, and n = 6 for all groups
cAMP content in lungs
The HIGH STRESS+/+ lungs had no significant increase in cAMP levels compared with LOW STRESS and NO STRESS controls. However, the HIGH STRESS−/− lungs had significantly increased cAMP levels versus HIGH STRESS+/+, LOW STRESS, and NO STRESS (p < 0.05; Fig. 5).
Lung cAMP content after 3 h of mechanical ventilation. Experimental groups are identical to those described in Fig. 1, and n = 5 for all groups. *HIGH STRESS−/− was significantly different from all other groups
MPO content in lungs
Lung MPO concentration was not different between wild-type and knockout in NO STRESS, LOW STRESS, and HIGH STRESS ventilation, but were each significantly lower than the CLP (positive control; p < 0.05) (ESM Fig. 4).
Effect of PI3Kγ kinase-dependent and kinase-independent inhibition
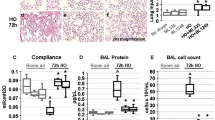
We used the inhibitors AS605240 to block the PI3Kγ kinase activity and enoximone, a PDE3 inhibitor, to mimic the increased cAMP resulting from the lack of PI3Kγ kinase-independent activity [20]. Wild-type lungs treated with both inhibitors showed significantly higher compliance compared with single inhibitor administration or vehicle-treated lungs (p < 0.05; Fig. 6a). Moreover, this compliance was similar to PI3Kγ-knockout lungs (Fig. 6a). Lungs treated with either AS605240 or enoximone had significantly lower BAL protein concentration compared with wild type (p < 0.05), and lungs treated with both inhibitors showed BAL protein concentration similar to knockout lungs (Fig. 6b).
Protective effects of AS605240 and enoximone on the lungs during HIGH STRESS mechanical ventilation. Experimental groups are HIGH STRESS+/+, HIGH STRESS+/+ plus AS605240, HIGH STRESS+/+ plus enoximone, HIGH STRESS+/+ plus AS605240 and enoximone, and HIGH STRESS−/−, where n = 5 for all groups. a Lung compliance. b BAL protein concentrations. c Nitrate/nitrite concentration in lung perfusate. d Lung cAMP content. *Significant difference with p < 0.05 compared with HIGH STRESS+/+. **Significant difference with p < 0.001 compared with HIGH STRESS+/+
Administration of the inhibitors was also associated with reduced pAkt lung expression, reduced nitrate/nitrite release, and increased cAMP levels. Lungs treated with AS605240 only or in combination with enoximone showed a significant reduction (p < 0.05) of pAkt lung expression (ESM Fig. 8) and nitrate/nitrite release (Fig. 6c) compared with wild-type lungs, with comparable levels to knockout lungs (ESM Figs. 8 and 6c). Moreover, lungs treated with enoximone only or in combination with AS605240 showed increased (p < 0.05) cAMP levels compared with wild-type lungs, with similar levels to knockout lungs (Fig. 6d). There were no differences among all inhibitor study groups during LOW STRESS ventilation (ESM Fig. 6). Enoximone had no effect when administered to PI3Kγ-knockout lungs during HIGH STRESS mechanical ventilation (ESM Fig. 7).
Discussion
The major finding of this study is that, in the absence of circulating leukocytes, PI3Kγ of resident lung cells directly contributes to the development of ventilation-induced lung injury (VILI) and stretch-induced alveolar edema. Furthermore, pharmacological blockade of PI3Kγ kinase activity alone or in combination with a therapy aimed to increase cAMP levels may be a suitable strategy to attenuate VILI.
The isolated and perfused lung model provided necessary advantages in that it: (1) mimics normal circulation and hemodynamic forces, (2) maintains pulmonary viability and function, and (3) allows for analysis of lung-derived mediator production [23]. Nonetheless, this model has some weaknesses. First, we assumed that our ventilated, perfused ex vivo lung model has a minimum number of neutrophils, which hence do not play a role in the increase in lung injury observed during HIGH STRESS ventilation. This assumption is based on the fact that the perfusate used is a cell-free solution that effectively “washes out” intravascular cells. However, to ensure that marginated and/or resident macrophages were not playing a major role, MPO and macrophage infiltration was evaluated, showing no differences between genotypes, strongly suggesting that inflammatory cells did not play a significant role. Finally, while this model was ideally suited to answer the questions posed in this study, the in vivo response may vary due to circulating leukocytes and inflammatory mediators.
PI3Kγ and its downstream target Akt influence migration, inflammation, and survival [18]. PI3Kγ also possesses kinase-independent scaffolding activity, which regulate intracellular cAMP concentrations [20, 21]. We previously demonstrated that PI3Kγ-knockout lungs were protected from VILI through modulation of the apoptosis/necrosis balance [22]. The novelty of this study specifically lies in the novel role of PI3Kγ in VILI-induced edema formation related to its kinase-dependent and kinase-independent activities. Additionally, we demonstrate that blocking PI3Kγ kinase activity and increasing lung cAMP levels may be a useful strategy to mitigate VILI.
During VILI, pulmonary edema occurs through two different mechanisms: tensile failure in the endothelial–epithelial barrier, and inhibition of alveolar fluid clearance [28, 29]. Since PI3Kγ may be involved in both of these mechanisms, we examined signaling pathways known to contribute to these systems.
The nitric oxide signaling pathway contributes to increased endothelial–epithelial barrier breakdown, vascular permeability, and edema formation during ventilation [10, 30, 31]. Additionally, eNOS activation is dependent on PI3K/Akt during mechanical ventilation [32]; therefore, we evaluated the effects of pulmonary PI3Kγ on nitric oxide synthesis alteration and byproduct production. We found that pAkt and peNOS, but not iNOS, were diminished in pulmonary tissue of PI3Kγ-knockout mice following HIGH STRESS ventilation. However, contrary to these findings, mice overexpressing eNOS showed attenuated VILI, which may result from the in vivo role of eNOS-derived NO in inhibiting leukocyte infiltration [33].
Specific downstream activation of PI3K pathways may alter the effect on vascular permeability, since activation of Src family kinase by PI3K increased permeability while PI3K/Akt/glycogen synthase kinase 3β activation limited lung edema [34]. Our findings provide new insights into the effects of PI3Kγ on NO and cAMP levels relative to edema formation during high-stress mechanical ventilation.
Nitric oxide has an extremely short half-life; however, the quantity of NO can be approximated by analysis of nitrate/nitrite, which are formed following its reaction with oxygen. NO can also induce production of peroxynitrite, which is a potent oxidant capable of damaging alveolar epithelium [13]. We found increased production of nitrate/nitrite and nitrotyrosine, a marker of peroxynitrite, in response to HIGH STRESS ventilation, which was completely abrogated in lungs lacking PI3Kγ. Several previous studies have correlated nitrotyrosine with the development of acute lung injury in patients [14, 35]. Therefore, we speculate that, since PI3Kγ-knockout lungs are protected from pulmonary nitrosylation and VILI, the PI3Kγ isoform plays a pivotal role in the loss of endothelial–epithelial barrier integrity.
Both animal and human studies have demonstrated that high-stress ventilation leads to cytokine/chemokine release associated with subsequent lung damage known as biotrauma [23, 36–38]. In addition, mediator release can be further altered by nitric oxide signaling [30]. While we confirm that pulmonary cytokine production was increased throughout HIGH STRESS ventilation, it was not be significantly different between genotypes, despite differences in NO activity. This may be due to the fact that iNOS-derived NO, unaltered in our experiment, is more closely linked with biotrauma. Moreover, the rise in chemotactic cytokines and subsequent neutrophil infiltration significantly lags behind the early induction of increased vascular permeability [22].
In addition to altering barrier permeability, PI3Kγ may also affect alveolar fluid clearance, further contributing to edema. Nitric oxide can inhibit alveolar fluid clearance during high-stress mechanical ventilation [11, 39]. Additionally, high-tidal-volume ventilation can induce a sustained reduction in cAMP-dependent alveolar fluid clearance [11]. PI3Kγ is known to modulate cAMP concentrations independent of its kinase activity [20, 21]. Since in our model HIGH STRESS−/−, but not HIGH STRESS+/+, displayed a significant augmentation of cAMP, this mechanism may contribute to decreased vascular permeability and increased alveolar fluid clearance.
In other disease models, such as sepsis [40] and arthritis [27], pharmacological inhibition of PI3Kγ kinase activity has been shown to modulate inflammation and improve outcomes. This study is the first showing that blocking PI3Kγ kinase activity in resident lung cells attenuates VILI through reduction in NO release. Moreover, a combined strategy using enoximone to increase cAMP, thereby mimicking the lack of PI3Kγ kinase-independent function, further attenuated VILI to levels similar to those in PI3Kγ-knockout mice.
In conclusion, this study demonstrates that pulmonary-derived PI3Kγ significantly contributes to the development of ventilation-induced lung injury and pulmonary edema. Furthermore, we correlate the development of VILI and edema with eNOS-derived nitric oxide signaling and cAMP hydrolysis, suggesting that PI3Kγ contributes to pulmonary edema by increasing endothelial−epithelial permeability and/or reducing alveolar fluid clearance. Ultimately, this study identifies PI3Kγ as a possible therapeutic target in the treatment and/or prevention of VILI and edema. Moreover, a pharmacological strategy able to block the effects of PI3Kγ in residential lung cells can mitigate the deleterious effects of mechanical stress, hopefully with minimal effect on the innate immune response.
References
Slutsky AS (1999) Lung injury caused by mechanical ventilation. Chest 116:9S–15S
Dos Santos CC, Slutsky AS (2000) Invited review: mechanisms of ventilator-induced lung injury: a perspective. J Appl Physiol 89:1645–1655
Ranieri VM, Suter PM, Tortorella C, De Tullio R, Dayer JM, Brienza A, Bruno F, Slutsky AS (1999) Effect of mechanical ventilation on inflammatory mediators in patients with acute respiratory distress syndrome: a randomized controlled trial. JAMA 282:54–61
Lachmann RA, van Kaam AH, Haitsma JJ, Lachmann B (2007) High positive end-expiratory pressure levels promote bacterial translocation in experimental pneumonia. Intensive Care Med 33:1800–1804
Uhlig U, Haitsma JJ, Goldmann T, Poelma DL, Lachmann B, Uhlig S (2002) Ventilation-induced activation of the mitogen-activated protein kinase pathway. Eur Respir J 20:946–956
Matthay MA, Robriquet L, Fang X (2005) Alveolar epithelium: role in lung fluid balance and acute lung injury. Proc Am Thorac Soc 2:206–213
de Prost N, Roux D, Dreyfuss D, Ricard JD, Le Guludec D, Saumon G (2007) Alveolar edema dispersion and alveolar protein permeability during high volume ventilation: effect of positive end-expiratory pressure. Intensive Care Med 33:711–717
Broccard AF, Feihl F, Vannay C, Markert M, Hotchkiss J, Schaller MD (2004) Effects of L-NAME and inhaled nitric oxide on ventilator-induced lung injury in isolated, perfused rabbit lungs. Crit Care Med 32:1872–1878
Peng X, Abdulnour RE, Sammani S, Ma SF, Han EJ, Hasan EJ, Tuder R, Garcia JG, Hassoun PM (2005) Inducible nitric oxide synthase contributes to ventilator-induced lung injury. Am J Respir Crit Care Med 172:470–479
Choi WI, Quinn DA, Park KM, Moufarrej RK, Jafari B, Syrkina O, Bonventre JV, Hales CA (2003) Systemic microvascular leak in an in vivo rat model of ventilator-induced lung injury. Am J Respir Crit Care Med 167:1627–1632
Frank JA, Pittet JF, Lee H, Godzich M, Matthay MA (2003) High tidal volume ventilation induces NOS2 and impairs cAMP- dependent air space fluid clearance. Am J Physiol 284:L791–L798
Ader F, Le Berre R, Lancel S, Faure K, Viget NB, Nowak E, Neviere R, Guery BP (2007) Inhaled nitric oxide increases endothelial permeability in Pseudomonas aeruginosa pneumonia. Intensive Care Med 33:503–510
Beckman JS, Beckman TW, Chen J, Marshall PA, Freeman BA (1990) Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA 87:1620–1624
Haddad IY, Pataki G, Hu P, Galliani C, Beckman JS, Matalon S (1994) Quantitation of nitrotyrosine levels in lung sections of patients and animals with acute lung injury. J Clin Invest 94:2407–2413
Zsengeller ZK, Ross GF, Trapnell BC, Szabo C, Whitsett JA (2001) Adenovirus infection increases iNOS and peroxynitrite production in the lung. Am J Physiol 280:L503–L511
Michel CC, Curry FE (1999) Microvascular permeability. Physiol Rev 79:703–761
de Prost N, Dreyfuss D, Ricard JD, Saumon G (2008) Terbutaline lessens protein fluxes across the alveolo-capillary barrier during high-volume ventilation. Intensive Care Med 34:763–770
Hirsch E, Katanaev VL, Garlanda C, Azzolino O, Pirola L, Silengo L, Sozzani S, Mantovani A, Altruda F, Wymann MP (2000) Central role for G protein-coupled phosphoinositide 3-kinase gamma in inflammation. Science 287:1049–1053
Uhlig U, Fehrenbach H, Lachmann RA, Goldmann T, Lachmann B, Vollmer E, Uhlig S (2004) Phosphoinositide 3-OH kinase inhibition prevents ventilation-induced lung cell activation. Am J Respir Crit Care Med 169:201–208
Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, Marengo S, Russo G, Azzolino O, Rybalkin SD, Silengo L, Altruda F, Wetzker R, Wymann MP, Lembo G, Hirsch E (2004) PI3 Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell 118:375–387
Perino A, Ghigo A, Damilano F, Hirsch E (2006) Identification of the macromolecular complex responsible for PI3 Kgamma-dependent regulation of cAMP levels. Biochem Soc Trans 34:502–503
Lionetti V, Lisi A, Patrucco E, De Giuli P, Milazzo MG, Ceci S, Wymann M, Lena A, Gremigni V, Fanelli V, Hirsch E, Ranieri VM (2006) Lack of phosphoinositide 3-kinase-gamma attenuates ventilator-induced lung injury. Crit Care Med 34:134–141
von Bethmann AN, Brasch F, Nusing R, Vogt K, Volk HD, Muller KM, Wendel A, Uhlig S (1998) Hyperventilation induces release of cytokines from perfused mouse lung. Am J Respir Crit Care Med 157:263–272
Ranieri VM, Zhang H, Mascia L, Aubin M, Lin CY, Mullen JB, Grasso S, Binnie M, Volgyesi GA, Eng P, Slutsky AS (2000) Pressure-time curve predicts minimally injurious ventilatory strategy in an isolated rat lung model. Anesthesiology 93:1320–1328
Muscedere JG, Mullen JB, Gan K, Slutsky AS (1994) Tidal ventilation at low airway pressures can augment lung injury. Am J Respir Crit Care Med 149:1327–1334
Martin EL, Sheikh TA, Leco KJ, Lewis JF, Veldhuizen RA (2007) Contribution of alveolar macrophages to the response of the TIMP-3 null lung during a septic insult. Am J Physiol 293:L779–L789
Camps M, Ruckle T, Ji H, Ardissone V, Rintelen F, Shaw J, Ferrandi C, Chabert C, Gillieron C, Francon B, Martin T, Gretener D, Perrin D, Leroy D, Vitte PA, Hirsch E, Wymann MP, Cirillo R, Schwarz MK, Rommel C (2005) Blockade of PI3 Kgamma suppresses joint inflammation and damage in mouse models of rheumatoid arthritis. Nat Med 11:936–943
Eckle T, Grenz A, Laucher S, Eltzschig HK (2008) A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J Clin Invest 118:3301–3315
Mehta D, Malik AB (2006) Signaling mechanisms regulating endothelial permeability. Physiol Rev 86:279–367
Moncada S, Higgs A (1993) The L-arginine-nitric oxide pathway. N Engl J Med 329:2002–2012
Song W, Matalon S (2007) Modulation of alveolar fluid clearance by reactive oxygen-nitrogen intermediates. Am J Physiol 293:L855–L858
Kuebler WM, Uhlig U, Goldmann T, Schael G, Kerem A, Exner K, Martin C, Vollmer E, Uhlig S (2003) Stretch activates nitric oxide production in pulmonary vascular endothelial cells in situ. Am J Respir Crit Care Med 168:1391–1398
Takenaka K, Nishimura Y, Nishiuma T, Sakashita A, Yamashita T, Kobayashi K, Satouchi M, Ishida T, Kawashima S, Yokoyama M (2006) Ventilator-induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. Am J Physiol 290:L1078–L1086
Miyahara T, Hamanaka K, Weber DS, Drake DA, Anghelescu M, Parker JC (2007) Phosphoinositide 3-kinase, Src, and Akt modulate acute ventilation-induced vascular permeability increases in mouse lungs. Am J Physiol 293:L11–L21
Kooy NW, Royall JA, Ye YZ, Kelly DR, Beckman JS (1995) Evidence for in vivo peroxynitrite production in human acute lung injury. Am J Respir Crit Care Med 151:1250–1254
Tremblay LN, Slutsky AS (1998) Ventilator-induced injury: from barotrauma to biotrauma. Proc Assoc Am Physicians 110:482–488
Chiumello D, Pristine G, Slutsky AS (1999) Mechanical ventilation affects local and systemic cytokines in an animal model of acute respiratory distress syndrome. Am J Respir Crit Care Med 160:109–116
Tremblay L, Valenza F, Ribeiro SP, Li J, Slutsky AS (1997) Injurious ventilatory strategies increase cytokines and c-fos m-RNA expression in an isolated rat lung model. J Clin Invest 99:944–952
Kaestle SM, Reich CA, Yin N, Habazettl H, Weimann J, Kuebler WM (2007) Nitric oxide-dependent inhibition of alveolar fluid clearance in hydrostatic lung edema. Am J Physiol 293:L859–L869
Martin EL, Souza DG, Fagundes CT, Amaral FA, Assenzio B, Puntorieri V, Del Sorbo L, Fanelli V, Bosco M, Delsedime L, Pinho JF, Lemos VS, Souto FO, Alves-Filho JC, Cunha FQ, Slutsky AS, Ruckle T, Hirsch E, Teixeira MM, Ranieri VM (2010) PI3Kγ kinase activity contributes to sepsis and organ damage by altering neutrophil recruitment. Am J Respir Crit Care Med, online first
Acknowledgments
We thank Drs. Paolo Provero and Claudia Filippini for statistical consultation. This study was supported by the Italian Ministry of University and Research PRIN 2002, ex 60% 2007, The Sixth Framework Programme EUGeneheart, and Foundation Leducq.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fanelli, V., Puntorieri, V., Assenzio, B. et al. Pulmonary-derived phosphoinositide 3-kinase gamma (PI3Kγ) contributes to ventilator-induced lung injury and edema. Intensive Care Med 36, 1935–1945 (2010). https://doi.org/10.1007/s00134-010-2018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-010-2018-y