Abstract
Purpose
To evaluate the effects of frequency and inspiratory plateau pressure (Pplat) during recruitment manoeuvres (RMs) on lung and distal organs in acute lung injury (ALI).
Methods
We studied paraquat-induced ALI rats. At 24 h, rats were anesthetized and RMs were applied using continuous positive airway pressure (CPAP, 40 cmH2O/40 s) or three-different sigh strategies: (a) 180 sighs/h and Pplat = 40 cmH2O (S180/40), (b) 10 sighs/h and Pplat = 40 cmH2O (S10/40), and (c) 10 sighs/h and Pplat = 20 cmH2O (S10/20).
Results
S180/40 yielded alveolar hyperinflation and increased lung and kidney epithelial cell apoptosis as well as type III procollagen (PCIII) mRNA expression. S10/40 resulted in a reduction in epithelial cell apoptosis and PCIII expression. Static elastance and alveolar collapse were higher in S10/20 than S10/40.
Conclusions
The reduction in sigh frequency led to a protective effect on lung and distal organs, while the combination with reduced Pplat worsened lung mechanics and histology.
Similar content being viewed by others
Introduction
The use of low tidal volumes and limited inspiratory plateau pressure has been proposed to ventilate acute respiratory distress syndrome (ARDS) and acute lung injury (ALI) patients and prevent lung and distal organ injury [1–4]. The reduction in tidal volume may result in alveolar derecruitment if not enough positive end-expiratory pressure (PEEP) is applied to prevent alveolar collapse [5]. On the other hand, high PEEP levels may be associated with excessive lung parenchyma stress and strain [6] and negative hemodynamic effects resulting in systemic organ injury [7].
Several recruitment manoeuvres (RMs) have been used in clinical and experimental ALI/ARDS to open the lung [8, 9]. In this line, continuous positive airway pressure (CPAP) of 40 cmH2O for 40 s (RM-CPAP) is a well-known method of RM since it improved respiratory function in several experimental models of ALI and in patients with ALI/ARDS. However, RM-CPAP failed to induce a sustained improvement of oxygenation [10, 11] and may result in lung injury [12, 13]. In this line, a RM at a frequency of 180 sighs/h (3 sighs/min) showed a beneficial [14, 15] but short-lived effect [16] on lung mechanics and oxygenation. Additionally, RM with high frequency and inspiratory plateau pressure may yield shear and tensile stresses resulting in lung damage [17]. There may be a certain level of frequency and inspiratory plateau pressure during RM which maintains the benefits on pulmonary function with less lung and distal organ injury.
In this study, we hypothesized that RM with high frequency and inspiratory plateau pressure may yield lung parenchyma and distal organ injury in healthy and ALI rats. We evaluated the effects of different levels of frequency and inspiratory plateau pressure during RM on arterial blood gases, lung static elastance and histology (light and electron microscopy), lung and distal organ epithelial cell apoptosis, and type III procollagen mRNA expression in lung tissue (an early marker of lung parenchyma remodelling). Some of the results of this study have been previously reported in abstract form [18].
Materials and methods
Detailed methods are described in the Electronic supplementary material (ESM) accompanying this article, and briefly summarized here.
Animal preparation and experimental protocol
This study was approved by the Ethics Committee of the Carlos Chagas Filho Institute of Biophysics, Health Sciences Centre, Federal University of Rio de Janeiro. Eighty Wistar rats (250–300 g) were randomly assigned to two groups. In Control (CTRL, n = 40), sterile saline solution (0.9% NaCl, 1.0 ml) was intraperitoneally (ip) injected and in acute lung injury (ALI, n = 40), paraquat (15 mg/kg, ip) was administered.
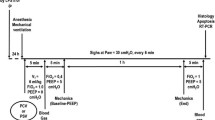
Twenty-four hours after saline or paraquat administration, animals were sedated (diazepam 1 mg, ip), anaesthetised (thiopental sodium 20 mg/kg, ip), tracheotomised, paralysed (pancuronium 2 mg/kg intravenously), and mechanically ventilated (Samay VR15, Universidad de la Republica, Montevideo, Uruguay) with the following parameters: tidal volume (V T) = 4 ml/kg, airflow = 6 ml/s, respiratory rate (RR) = 100 breaths/min, inspiratory to expiratory ratio = 1:2, fraction of inspired oxygen (FiO2) = 0.21, and PEEP = 5 cmH2O. The dosage, duration and level of anaesthesia were similar in all groups. A polyethylene catheter was introduced into the femoral artery to collect blood sampling and measure arterial pressure. CTRL and ALI animals were then randomized as follows: (1) non-recruited (NR) (n = 8/group); (2) recruitment manoeuvre (RM) consisting of one continuous positive airway pressure of 40 cmH2O for 40 s (RM-CPAP) (n = 8/group), (3) RM with 180 sighs/h (3 sighs/min) and inspiratory plateau pressure (Pplat) of 40 cmH2O (S180/40) (n = 8/group), (4) RM with 10 sighs/h (one sigh every 6 min) and Pplat of 40 cmH2O (S10/40) (n = 8/group), or (5) RM with 10 sighs/h and Pplat of 20 cmH2O (S10/20) (n = 8/group). We delivered a sequence of 180 sighs/h, in volume control mode, since it is the closest to the clinical setting [14, 15]. The other levels of pressure or frequency were decided based on some pilot studies. All experiments were performed at the same time. The frequency of non-sigh tidal volume was reduced to maintain minute ventilation constant among groups. Before randomization (BASELINE) and after 1-h ventilation period (END), arterial blood gases (i-STAT, Abbott Laboratories, IL, USA) and lung static elastance were analysed. After ventilation period, animals were then killed, lungs and distal organs were prepared for histology, and type III procollagen (PCIII) mRNA expression in lung tissue was measured.
Mechanics
Airflow, tracheal and oesophageal pressures were measured. Tidal volume (V T) was calculated by digital integration of flow signal. Transpulmonary pressures were calculated by the difference between tracheal and oesophageal pressures [13]. To calculate lung static elastance (Est,L), airways were occluded at end-inspiration until a transpulmonary plateau pressure (Pplat,L) was reached (at the end of 5 s), after which this value was divided by V T [13].
Histology
Light microscopy
A laparotomy was done immediately after the determination of lung mechanics (END). Lungs, liver, kidneys, and small intestine were removed, fixed in 3% buffered formaldehyde, paraffin embedded, and stained with haematoxylin–eosin. The volume fraction of the lung occupied by hyperinflated structures (alveolar ducts, alveolar sacs or alveoli wider than 120 μm) or collapsed alveoli or normal pulmonary areas were determined by the point-counting technique [13, 19].
Transmission electron microscopy
Three slices 2 × 2 × 2 mm were cut from three different segments of the left lung and fixed for electron microscopy analysis. The following structural damages were analyzed: (a) alveolar capillary membrane, (b) type II epithelial cells, and (c) endothelial cells, and then graded according to a 5-point semi-quantitative severity-based scoring system: 0 = normal lung parenchyma, 1 = changes in 1–25%, 2 = changes in 26–50%, 3 = changes in 51–75%, and 4 = changes in 76–100% of examined tissue [13]. Two investigators, unaware of the origin of the material, examined the samples microscopically. The slides were coded and examined only at the end of all measurements.
Apoptosis assay of lung and distal organs
Apoptotic cells of lung, kidney, liver, and small intestine villi were quantified using Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay [20] and immunohistochemical staining for Fas and FasL protein [21] in a blinded fashion by two pathologists. A 5-point semiquantitative severity-based scoring system was used and graded as: 0 = no apoptotic cells; 1 = 1–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100% of apoptotic cells in the examined tissue.
Semi-quantitative reverse-transcription and polymerase chain reaction
Lung parenchyma strips (3 × 3 × 10 mm) were longitudinally cut from left lungs. Total RNA was isolated from the frozen lung tissue and the relative expression of PCIII was obtained by semi-quantitative Reverse-Transcription and Polymerase Chain Reaction (RT-PCR). In the PCIII mRNA detection by RT-PCR, the rat glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) primers were used as internal positive control [12, 13].
Statistical analysis
The normality of the data (Kolmogorov–Smirnov test with Lilliefors’ correction) and the homogeneity of variances (Levene median test) were tested. If both conditions were satisfied, the effects of different ventilatory strategies in CTRL and ALI groups were analysed by using Two-way ANOVA followed by Tukey’s test. Otherwise, two-way ANOVA on ranks followed by Dunn’s post hoc test was selected instead. These two tests were used to compare lung histological data and PCIII mRNA expression. The effects of ventilatory strategies on CTRL and ALI groups at BASELINE and END (time) were assessed by using a three-way ANOVA with repeated measures on the time factor. This test was used to compare lung static elastance and arterial blood gases. The significance level was set at 5%. The parametric data were expressed as mean ± SEM, while the non-parametric data were expressed as median (Interquartile range). All tests were performed using SigmaStat 3.0 statistical software package (Jandel Corporation, San Raphael, CA, USA).
Results
Mean arterial pressure was maintained stable and at adequate levels during the experiments (see ESM).
In ALI, PaO2 and pH were lower while PaCO2 was higher than in CTRL (Table 1). The percentage of increase of PaO2 from BASELINE to END was 13 and 48% after RM-CPAP and S180/40, respectively (Table 1). S10/40 and S10/20 groups presented greater increase in PaO2 (58 and 54%, respectively) compared to S180/40. PaCO2 and pH were lower in S180/40 and S10/40 compared to the other groups with no significant changes between them (Table 1).
As shown in Fig. 1, Est,L significantly increased in ALI compared to CTRL. RM-CPAP did not modify Est,L while sigh (S180/40, S10/40, and S10/20) significantly decreased Est,L. Est,L was lower in S10/40 than S10/20.
Lung static elastance (Est,L) immediately before (BASELINE) and 1 h after (END) recruitment manoeuvre (RM) in control (CTRL) and acute lung injury (ALI). CTRL and ALI groups were randomized as follows: (a) non-recruited (NR), (b) a 40-s inflation to a peak airway pressure of 40 cmH2O (RM-CPAP), (c) 180 sighs/h and plateau pressure of 40 cmH2O (S180/40), (d) 10 sighs/h and plateau pressure of 40 cmH2O (S10/40), and (e) 10 sighs/h and a plateau pressure of 20 cmH2O (S10/20). Values are mean ± SEM of eight animals in each group (ten determinations per animal). *Significantly different from BASELINE (P < 0.05). †Significantly different from NR group (P < 0.05). ‡Significantly different from RM-CPAP group (P < 0.05). **All data from ALI groups were significantly different from CTRL (P < 0.05)
The fraction area of alveolar collapse was higher in ALI compared to CTRL. S180/40 showed a reduction of alveolar collapse and increased hyperinflated areas compared to RM-CPAP (Fig. 2). S10/40 led to a decrease in alveolar collapse and hyperinflation, while the amount of alveolar collapse was higher in S10/20 than S10/40 (Fig. 2).
The volume fraction of the lung occupied by hyperinflated structures (dark gray) or collapsed alveoli (gray) or normal pulmonary areas (white) in control (CTRL) and acute lung injury (ALI) groups. All values were computed in ten random, non-coincident fields per rat. Values are mean ± SEM of eight animals in each group. CTRL and ALI groups were randomized as follows: (a) non-recruited (NR), (b) a 40-s inflation to a peak airway pressure of 40 cmH2O (RM-CPAP), (c) 180 sighs/h and plateau pressure of 40 cmH2O (S180/40), (d) 10 sighs/h and plateau pressure of 40 cmH2O (S10/40), and (e) 10 sighs/h and a plateau pressure of 20 cmH2O (S10/20). *Significantly different from CTRL-NR (P < 0.05). †Significantly different from NR group (P < 0.05). ‡Significantly different from RM-CPAP group (P < 0.05). #Significantly different from S180/40 (P < 0.05). §Significantly different from S10/40 (P < 0.05). **All data from ALI groups were significantly different from CTRL (P < 0.05)
CTRL animals showed no lung ultrastructural modifications independent of RM. All ALI animals presented cytoplasmatic degeneration of type II pneumocyte (PII) and endothelial damage (Table 2). However, in S180/40, type II pneumocyte and endothelial damage was higher with a detachment of alveolar epithelium and denudation of epithelial basement membrane. In S10/40 and S10/20 these ultrastructural changes were minimized (Table 2).
The present model of ALI led to lung and distal organ epithelial cell apoptosis, while in CTRL the amount of epithelial cell apoptosis in lung and distal organs was similar among the groups. Lung and kidney apoptosis were increased only in S180/40 (Table 3). Photomicrographs of light and electron microscopy and immunohistochemistry are shown in ESM.
PCIII mRNA expression was higher in ALI than CTRL. In both ALI and CTRL, S180/40 increased PCIII mRNA expression (Fig. 3) while RM-CPAP caused no alteration. PCIII mRNA expression was significantly lower in S10/40 compared to S180/40, with no significant difference between S10/40 and S10/20 (Fig. 3).
Relative expression of type III procollagen mRNA (PCIII) obtained by amplification of PCIII and glyceraldehydes-3-phosphate-dehydrogenase (GAPDH) by semi-quantitative reverse-transcription and polymerase chain reaction (RT-PCR) of rat lung tissue in control (CTRL) and acute lung injury (ALI) groups. CTRL and ALI groups were randomized as follows: (a) non-recruited (NR), (b) a 40-s inflation to a peak airway pressure of 40 cmH2O (RM-CPAP), (c) 180 sighs/h and plateau pressure of 40 cmH2O (S180/40), (d) 10 sighs/h and plateau pressure of 40 cmH2O (S10/40), and (e) 10 sighs/h and a plateau pressure of 20 cmH2O (S10/20). Values are mean ± SEM (n = 4) of the ratio between the densitometric values of PCIII and GAPDH bands obtained in RT-PCR experiments. †Significantly different from NR group (P < 0.05). ‡Significantly different from RM-CPAP group (P < 0.05). #Significantly different from S180/40 (P < 0.05). §Significantly different from S10/40 (P < 0.05). **All data from ALI groups were significantly different from CTRL (P < 0.05)
Discussion
In the present experimental model of moderate ALI, a RM with standard sigh [180 sighs/h and Pplat = 40 cmH20 (S180/40)] improved oxygenation and decreased PaCO2, Est,L, and alveolar collapse; nevertheless, it yielded hyperinflation, ultrastructural changes in alveolar capillary membrane, increased lung and kidney epithelial cell apoptosis, and PCIII mRNA expression in lung tissue. On the other hand, RM with 10 sighs/h and Pplat = 40 cmH20 (S10/40) diminished Est,L and improved oxygenation, with a marked decrease in alveolar hyperinflation, PCIII mRNA expression in lung tissue, and apoptosis in lung and kidney epithelial cells. However, this sigh frequency associated with a lower Pplat of 20 cmH2O (S10/20) worsened Est,L, histology and oxygenation, increased PaCO2 but did not modify PCIII mRNA expression in lung tissue and epithelial cells apoptosis of distal organs. Therefore, RM with high frequency or low plateau pressure should be avoided.
To our knowledge this is the first study investigating the role of frequency and Pplat during RMs on lung and distal organ injury in an experimental model of ALI.
ALI was induced by paraquat, a herbicide which yields a moderate lung injury [22] with epithelial cell apoptosis in distal organs [23]. In our study the degree of alveolar collapse in non-recruited (NR) ALI was around 26% (Fig. 2) similar to that observed in previous experimental [12, 22] and human studies in ALI/ARDS [24]. After 1-h ventilation, Est,L increased while oxygenation decreased in NR animals. This deterioration may be caused by the use of limited V T and/or an insufficient PEEP level to open collapsed alveoli. We decided to use low V T and 5 cmH2O PEEP to minimize the possible interactions between conventional mechanical ventilation and different recruitment strategies on ventilator-induced lung injury (VILI) [25, 26]. Besides, animals were ventilated in air to prevent reabsorption atelectasis [27] and reduce possible hyperoxia-induced lung injury [28].
Comparison between RM-CPAP and S180/40
RM-CPAP did not significantly change Est,L, but improved oxygenation and reduced atelectasis after 1-h ventilation period. We cannot rule out the fact that RM-CPAP may lead to an early improvement in Est,L and its beneficial effects wore off with time. Furthermore, the association of 5 cmH2O PEEP with V T of 4 ml/kg may preclude positive effects of 1-h mechanical ventilation.
Est,L and the amount of alveolar collapse was lower while PaO2 higher in S180/40 compared to RM-CPAP, in accordance with previous studies in ALI/ARDS patients both in supine [14] and prone positioning [15]. In contrast, S180/40 group presented alveolar hyperinflation, severe epithelial and endothelial injury (Table 2), associated with an increase in PCIII mRNA expression (Fig. 3). The high pressure of sigh used to re-expand and open collapsed lung units may expose the alveoli to tensile and shear stresses stimulating fibroblasts and macrophages to synthesize collagen fibres [29, 30]. Our results are in accordance with previous reports demonstrating increased procollagen mRNA expression in lungs submitted to high airway pressures [31, 32], high inflation [12, 13, 32] or cyclic mechanical strain [30]. Additionally, sigh led to higher lung and kidney epithelial cell apoptosis, in line with other studies, which reported epithelial cell apoptosis in distal organs during injurious mechanical ventilation [7, 33]. In this context, kidney has been described as the first distal organ to be damaged during injurious mechanical ventilation [34, 35]. Distal organ injury process may be related to the (1) release of circulating soluble Fas ligand and cytokines [7] and/or (2) hypotension or depressed cardiac function resulting in decreased organ perfusion. Although mean arterial pressure was maintained at adequate levels during the experiments, we cannot rule out possible cardiac output reduction. Furthermore, even though we did not measure FasL or cytokines, lung and kidney apoptosis may be associated with increased PCIII expression. Therefore, we may hypothesize that the release of inflammatory mediators induced by excessive pulmonary stress and strain could have contributed to lung and kidney injury. However, the degree of lung stress induced by sigh was probably not high enough to cause small intestine villi and liver epithelial cell apoptosis [36]. We also found an important dissociation between the improvement in the clinical parameters, i.e. oxygenation and lung elastance, and molecular (Fig. 3) and ultra-structural (Table 2) damages in lung parenchyma. This observation is important since the measurements of oxygenation and lung mechanics are most commonly used to optimize mechanical ventilation at the bedside; however, our experimental data suggest that they do not necessarily represent the optimal parameters to monitor the possible RMs effects on lung and peripheral organ injury [25].
Reduction of frequency during sigh
Higher respiratory frequencies may induce lung injury by both elevating the magnitude of shear stress from more rapid inflations (thereby exceeding the failure limit and reducing the number of cycles required for failure) and more rapidly achieving the total number of cycles required for failure [37, 38]. Additionally, the increase in respiratory frequency has been reported to be associated with the release of inflammatory mediators [39]. Therefore, lower sigh frequency resulted in less lung epithelial and endothelial damage, leading to reduced PCIII expression and lung epithelial cell apoptosis. We speculate that there is a sigh frequency threshold beyond which the intrinsic reparative properties of the lung epithelium are overwhelmed. Clearly, the optimal sigh frequency may be different in healthy and ALI animals/patients.
We also observed a reduction in kidney epithelial cell apoptosis that could be related to the attenuation of the overall inflammatory process [17]. On the other hand, we cannot rule out the possible improvement of regional perfusion during reduced sigh frequency, leading to better oxygen delivery to peripheral organs.
Reduction of plateau pressure during sigh
The use of a reduced inspiratory Pplat of 20 cmH2O was based on some pilot studies which showed that PCIII mRNA expression remained higher in S10/40 than NR-ALI animals. This level of Pplat is approximately double the mean Pplat achieved during conventional tidal volume in this experimental ALI model. We found that the association of reduced sigh frequency and Pplat (S10/20) worsened oxygenation, lung mechanics and histology with no significant modification in epithelial cell apoptosis of the lung and distal organs, and PCIII mRNA expression. Thus, although lower Pplat during sigh reduced the tensile stress, it was unable to open the collapsed alveoli resulting in shear stress while maintaining higher PCIII mRNA expression [12].
Limitation of the study
The current study has several limitations: (1) we used a specific experimental model of moderate ALI induced by paraquat. Thus, we do not know if similar results can be obtained in other experimental models of ALI, in larger animals, with different degrees of lung injury, amount of recruitable tissue or consolidation; (2) the short duration of the experiment, just 1 h, which hinders assessment of possible long term effects of RMs; (3) different types of RMs have been proposed, with periodic increase in Pplat, PEEP or both [9, 17]. However, in the present study, we only evaluated sigh performed by periodic changes in Pplat at different frequencies. We cannot exclude that sigh frequencies lower than 10/h (S10/40) but higher than 1/h (RM-CPAP), may further improve respiratory function minimizing lung injury. Additionally, the reduction of sigh frequency under the same inspiratory plateau pressure reduced lung injury; (4) PEEP was not individually titrated. A fixed level of 5 cmH2O PEEP was applied to avoid the possible bias due to the interaction between different PEEP levels and RMs. Thus, we cannot exclude that different results could have been obtained at higher PEEP levels; (5) only PCIII mRNA expression was measured, therefore the impact of RMs on different inflammatory mediators was not evaluated; and (6) although mean arterial pressure remains stable and at adequate levels during the experiments, the association between reduced regional perfusion induced by RMs and distal organ damage cannot be ruled out.
Conclusion
This study, under the present experimental conditions, demonstrates that sigh at 40 cmH2O Pplat was effective at opening collapsed alveoli, improving oxygenation and lung mechanics independent of the frequency. The reduction in sigh frequency led to a better lung morphofunctional and molecular profile. However, the combination of lower sigh frequency and inspiratory plateau pressure worsened lung function and histology, with no further protective effects on lung and distal organs. The best method of recruitment manoeuvre remains uncertain and the optimal inspiratory plateau pressure, duration and periodicity need to be elucidated.
References
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR (1998) Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med 338:347–354
Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 342:1301–1308
Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, Davies AR, Hand LE, Zhou Q, Thabane L, Austin P, Lapinsky S, Baxter A, Russell J, Skrobik Y, Ronco JJ, Stewart TE, Lung Open Ventilation Study Investigators (2008) Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:637–645
Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, Lefrant JY, Prat G, Richecoeur J, Nieszkowska A, Gervais C, Baudot J, Bouadma L, Brochard L, Expiratory Pressure (Express) Study Group (2008) Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA 299:646–655
Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ (2001) Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med 164:122–130
Nieszkowska A, Lu Q, Vieira S, Elman M, Fetita C, Rouby JJ (2004) Incidence and regional distribution of lung overinflation during mechanical ventilation with positive end-expiratory pressure. Crit Care Med 32:1496–1503
Imai Y, Parodo J, Kajikawa O, de Perrot M, Fischer S, Edwards V, Cutz E, Liu M, Keshavjee S, Martin TR, Marshall JC, Ranieri VM, Slutsky AS (2003) Injurious mechanical ventilation and end-organ epithelial cell apoptosis and organ dysfunction in an experimental model of acute respiratory distress syndrome. JAMA 289:2104–2112
Piacentini E, Villagrá A, López-Aguilar J, Blanch L (2004) Clinical review: the implications of experimental and clinical studies of recruitment maneuvers in acute lung injury. Crit Care 8:115–121
Grasso S, Mascia L, Del Turco M, Malacarne P, Giunta F, Brochard L, Slutsky AS, Ranieri VM (2002) Effects of recruiting maneuvers in patients with acute respiratory distress syndrome ventilated with protective ventilatory strategy. Anesthesiology 96:795–802
Musch G, Harris RS, Vidal Melo MF, O’Neill KR, Layfield JD, Winkler T, Venegas JG (2004) Mechanism by which a sustained inflation can worsen oxygenation in acute lung injury. Anesthesiology 100:323–330
Oczenski W, Hörmann C, Keller C, Lorenzl N, Kepka A, Schwarz S, Fitzgerald RD (2004) Recruitment maneuvers after a positive end expiratory pressure trial do not induce sustained effects in early adult respiratory distress syndrome. Anesthesiology 101:620–625
Farias LL, Faffe DS, Xisto DG, Santana MC, Lassance R, Prota LF, Amato MB, Morales MM, Zin WA, Rocco PR (2005) Positive end-expiratory pressure prevents lung mechanical stress caused by recruitment/derecruitment. J Appl Physiol 98:53–61
Riva DR, Oliveira MB, Rzezinski AF, Rangel G, Capelozzi VL, Zin WA, Morales MM, Pelosi P, Rocco PR (2008) Recruitment maneuver in pulmonary and extrapulmonary experimental acute lung injury. Crit Care Med 36:1900–1908
Pelosi P, Cadringher P, Bottino N, Panigada M, Carrieri F, Riva E, Lissoni A, Gattinoni L (1999) Sigh in acute respiratory distress syndrome. Am J Respir Crit Care Med 159:872–880
Pelosi P, Bottino N, Chiumello D, Caironi P, Panigada M, Gamberoni C, Colombo G, Bigatello LM, Gattinoni L (2003) Sigh in supine and prone position during acute respiratory distress syndrome. Am J Respir Crit Care Med 167:521–527
Fujino Y, Goddon S, Dolhnikoff M, Hess D, Amato MB, Kacmarek RM (2001) Repetitive high-pressure recruitment maneuvers required to maximally recruit lung in a sheep model of acute respiratory distress syndrome. Crit Care Med 29:1579–1586
Allen GB, Suratt BT, Rinaldi L, Petty JM, Bates JH (2006) Choosing the frequency of deep inflation in mice: balancing recruitment against ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol 91:710–717
Steimback PW, Silva PL, Oliveira GP, Rangel G, Capelozzi VL, Morales MM, Pelosi P, Rocco PR (2007) Sigh induced lung mechanical stress in acute lung injury. Am J Resp Crit Care A22
Weibel ER (1990) Morphometry: stereological theory and practical methods. In: Gil J (ed) Models of lung disease—microscopy and structural methods. Marcel Dekker, New York, pp 199–247
Menezes SL, Bozza PT, Neto HC, Laranjeira AP, Negri EM, Capelozzi VL, Zin WA, Rocco PR (2005) Pulmonary and extrapulmonary acute lung injury: inflammatory and ultrastructural analyses. J Appl Physiol 98:1777–1783
Baptista AL, Parra ER, Filho JV, Kairalla RA, de Carvalho CR, Capelozzi VL (2006) Structural features of epithelial remodeling in usual interstitial pneumonia histologic pattern. Lung 184:239–244
Rocco PR, Negri EM, Kurtz PM, Vasconcellos FP, Silva GH, Capelozzi VL, Romero PV, Zin WA (2001) Lung tissue mechanics and extracellular matrix remodeling in acute lung injury. Am J Respir Crit Care Med 164:1067–1071
Fabisiak JP, Kagan VE, Tyurina YY, Tyurin VA, Lazo JS (1998) Paraquat-induced phosphatidylserine oxidation and apoptosis are independent of activation of PLA2. Am J Physiol 274:793–802
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G (2006) Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med 354:1775–1786
Chiumello D, Carlesso E, Cadringher P, Caironi P, Valenza F, Polli F, Tallarini F, Cozzi P, Cressoni M, Colombo A, Marini JJ, Gattinoni L (2008) Lung stress and strain during mechanical ventilation of the acute respiratory distress syndrome. Am J Respir Crit Care Med 178:346–355
Gattinoni L, Carlesso E, Cadringher P, Valenza F, Vagginelli F, Chiumello D (2003) Physical and biological triggers of ventilator-induced lung injury and its prevention. Eur Respir J 47:15s–25s
Rothen HU, Sporre B, Engberg G, Wegenius G, Reber A, Hedenstierna G (1995) Prevention of atelectasis during general anaesthesia. Lancet 345:1387–1391
Kulkarni AC, Kuppusamy P, Parinandi N (2007) Oxygen, the lead actor in the pathophysiologic drama: enactment of the trinity of normoxia, hypoxia, and hyperoxia in disease and therapy. Antioxid Redox Signal 9:1717–1730
Dreyfuss D, Saumon G (1998) Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med 157:294–323
Garcia CS, Rocco PR, Facchinetti LD, Lassance RM, Caruso P, Deheinzelin D, Morales MM, Romero PV, Faffe DS, Zin WA (2004) What increases type III procollagen mRNA levels in lung tissue: stress induced by changes in force or amplitude? Respir Physiol Neurobiol 144:59–70
de Carvalho ME, Dolhnikoff M, Meireles SI, Reis LF, Martins MA, Deheinzelin D (2007) Effects of overinflation on procollagen type III expression in experimental acute lung injury. Crit Care 11:R23
Berg JT, Fu Z, Breen EC, Tran HC, Mathieu-Costello O, West JB (1997) High lung inflation increases mRNA levels of ECM components and growth factors in lung parenchyma. J Appl Physiol 83:120–128
Nakos G, Batistatou A, Galiatsou E, Konstanti E, Koulouras V, Kanavaros P, Doulis A, Kitsakos A, Karachaliou A, Lekka ME, Bai M (2006) Lung and ‘end organ’ injury due to mechanical ventilation in animals: comparison between the prone and supine positions. Crit Care 10:R38
Ranieri VM, Giunta F, Suter PM, Slutsky AS (2000) Mechanical ventilation as a mediator of multisystem organ failure in acute respiratory distress syndrome. JAMA 284:43–44
Koyner JL, Murray PT (2008) Mechanical ventilation and lung–kidney interactions. Clin J Am Soc Nephrol 3:562–570
Martin TR (2008) Interactions between mechanical and biological processes in acute lung injury. Proc Am Thorac Soc 5:291–296
Conrad SA, Zhang S, Arnold TC, Scott LK, Carden DL (2005) Protective effects of low respiratory frequency in experimental ventilator-associated lung injury. Crit Care Med 33:835–840
Garcia CS, Abreu SC, Soares RM, Prota LF, Figueira RC, Morales MM, Capelozzi VL, Zin WA, Rocco PR (2008) Pulmonary morphofunctional effects of mechanical ventilation with high inspiratory air flow. Crit Care Med 36:232–239
Vaporidi K, Voloudakis G, Priniannakis G, Kondili E, Koutsopoulos A, Tsatsanis C, Georgopoulos D (2008) Effects of respiratory rate on ventilator-induced lung injury at a constant PaCO2 in a mouse model of normal lung. Crit Care Med 36:1277–1283
Acknowledgments
We would like to express our gratitude to Mr. Andre Benedito da Silva for animal care, Mrs. Miriam Regina Taborda Simone and Ana Lucia Neves da Silva for their help with microscopy, Ms. Jaqueline Lima do Nascimento for her skilful technical assistance during the experiments, and Mrs. Moira Elizabeth Schöttler for assistance in editing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was supported by Centres of Excellence Program (PRONEX-FAPERJ), Brazilian Council for Scientific and Technological Development (CNPq), Carlos Chagas Filho, Rio de Janeiro State Research Supporting Foundation (FAPERJ), São Paulo State Research Supporting Foundation (FAPESP).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Steimback, P.W., Oliveira, G.P., Rzezinski, A.F. et al. Effects of frequency and inspiratory plateau pressure during recruitment manoeuvres on lung and distal organs in acute lung injury. Intensive Care Med 35, 1120–1128 (2009). https://doi.org/10.1007/s00134-009-1439-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-009-1439-y