Abstract
Objective
Physiological track and trigger warning systems (TTs) are used to identify patients outside critical care areas at risk of deterioration and to alert a senior clinician, Critical Care Outreach Service, or equivalent. The aims of this work were: to describe published TTs and the extent to which each has been developed according to established procedures; to review the published evidence and available data on the reliability, validity and utility of existing systems; and to identify the best TT for timely recognition of critically ill patients.
Design and setting
Systematic review of studies identified from electronic, citation and hand searching, and expert informants. Cohort study of data from 31 acute hospitals in England and Wales.
Measurements and results
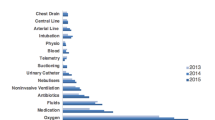
Thirty-six papers were identified describing 25 distinct TTs. Thirty-one papers described the use of a TT, and five were studies examining the development or testing of TTs. None of the studies met all methodological quality standards. For the cohort study, outcome was measured by a composite of death, admission to critical care, ‘do not attempt resuscitation’ or cardiopulmonary resuscitation. Fifteen datasets met pre-defined quality criteria. Sensitivities and positive predictive values were low, with median (quartiles) of 43.3 (25.4–69.2) and 36.7 (29.3–43.8), respectively.
Conclusion
A wide variety of TTs were in use, with little evidence of reliability, validity and utility. Sensitivity was poor, which might be due in part to the nature of the physiology monitored or to the choice of trigger threshold. Available data were insufficient to identify the best TT.



Similar content being viewed by others
References
Department of Health and NHS Modernisation Agency (2003) The National Outreach Report. Department of Health, London
Berwick DM, Calkins DR, McCannon CJ, Hackbarth AD (2006) The 100,000 Lives Campaign. JAMA 295:324–327
Gao H, Harrison DA, Adam S, Daly K, Goldhill DR, Parry GJ, Rashidian A, Subbe CP, Harvey S (2006) Evaluation of available data on physiological track and trigger warning systems. Crit Care 10(Suppl 1):P415
Lee A, Bishop G, Hillman KM, Daffurn K (1995) The Medical Emergency Team. Anaesth Intensive Care 23(2):183–186
Morgan RJM, Williams F, Wright MM (1997) An early warning scoring system for detecting developing critical illness. Clinical Intensive Care 8:100
Intensive Care Society (2002) Guidelines for the Introduction of Outreach Services. Intensive Care Society, London
Department of Health (2000) Comprehensive critical care: a review of adult critical care services. Department of Health, London
The Royal College of Physicians Working Party (2002) The interface between acute general medicine and critical care. Royal College of Physicians, London
Audit Commission (1999) Critical to success: the place of efficient and effective critical care services within the acute hospital. Audit Commission, London
Laupacis A, Sekar N, Stiell IG (1997) Clinical prediction rules: a review and suggested modifications of methodological standards. JAMA 277:488–494
Bossuyt PM, Reitsma JB, Bruns DE, Gatsonis CA, Glasziou PP, Irwig LM, Jeroen G. Lijmer JG, Moher D, Rennie D, de Vet HCW (2003) Towards complete and accurate reporting of studies of diagnostic accuracy: the STARD initiative. BMJ 326:41–44
McGinn TG, Guyatt GH, Wyer PC, Naylor CD, Stiell IG, Richardson WS (2000) Users' guides to the medical literature XXII: how to use articles about clinical decision rules. JAMA 284:79–84
Black N, Payne M, on behalf of the DoCDat Development Group (2003) Directory of Clinical Databases: improving and promoting their use. Qual Saf Health Care 12:348–352
Directory of Clinical Databases [http://www.docdat.org]
Whiting P, Rutjes AW, Reitsma JB, Bossuyt PM, Kleijnen J (2003) The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 3:25
Lijmer JG, Bossuyt MM, Heisterkamp SH (2002) Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med 21:1525–1537
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–1558
Buist M, Bernard S, Nguyen TV, Moore G, Anderson J (2004) Association between clinically abnormal observations and subsequent in-hospital mortality: a prospective study. Resuscitation 62:137–141
Goldhill DR, Worthington L, Mulcahy A, Tarling M, Sumner A (1999) The patient-at-risk team: identifying and managing seriously ill ward patients. Anaesthesia 54:853–860
Hodgetts TJ, Kenward G, Vlachonikolis IG, Payne S, Castle N (2002) The identification of risk factors for cardiac arrest and formulation of activation criteria to alert a medical emergency team. Resuscitation 54:125–131
Subbe CP, Kruger M, Rutherford P, Gemmel L (2001) Validation of a modified Early Warning Score in medical admissions. QJM 94:521–526
Goldhill DR, McNarry AF (2004) Physiological abnormalities in early warning scores are related to mortality in adult inpatients. Br J Anaesth 92:882–884
Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart GK, Opdam H, Silvester W, Doolan L, Gutteridge G (2003) A prospective before-and-after trial of a medical emergency team. Med J Aust 179:283–287
Bellomo R, Goldsmith D, Uchino S, Buckmaster J, Hart G, Opdam H, Silvester W, Doolan L, Gutteridge G (2004) Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med 32:916–921
Crispin C, Daffurn K (1998) Nurses' responses to acute severe illness. Aust Crit Care 11:131–133
Hillman KM, Bishop G, Lee A. Daffurn K, Bauman A, Crispin C, Ince L Bristow P, Hourihan F (1996) Identifying the general ward patient at high risk of cardiac arrest. Clinical Intensive Care 7:242–243
Hillman K, Parr M, Flabouris A, Bishop G, Stewart A (2001) Redefining in-hospital resuscitation: the concept of the medical emergency team. Resuscitation 48:105–110
Hillman K, Chen J, Brown D (2003) A clinical model for Health Services Research: the Medical Emergency Team. J Crit Care 18:195–199
Hourihan F, Bishop G, Hillman KM, Daffurn K, Lee A (1995) The Medical Emergency Team: a new strategy to identify and intervene in high-risk patients. Clinical Intensive Care 6:269–272
Lee A, Lum ME, O'Regan WJ, Hillman KM (1998) Early postoperative emergencies requiring an intensive care team intervention: the role of ASA physical status and after- hours surgery. Anaesthesia 53:529–535
Parr MJ, Hadfield JH, Flabouris A, Bishop G, Hillman K (2001) The Medical Emergency Team: 12 month analysis of reasons for activation, immediate outcome and not-for-resuscitation orders. Resuscitation 50:39–44
Bristow PJ, Hillman KM, Chey T, Daffurn K, Jacques TC, Norman SL, Bishop GF, Simmons EG (2000) Rates of in-hospital arrests, deaths and intensive care admissions: the effect of a medical emergency team. Med J Aust 173:236–240
Buist MD, Moore GE, Bernard SA, Waxman BP, Anderson JN, Nguyen TV (2002) Effects of a medical emergency team on reduction of incidence of and mortality from unexpected cardiac arrests in hospital: preliminary study. BMJ 324:387–390
Cioffi J (2000) Recognition of patients who require emergency assistance: a descriptive study. Heart Lung 29:262–268
Daly FFS, Sidney KL, Fatovich DM (1998) The Medical Emergency Team (MET): a model for the district general hospital. Aust N Z J Med 28:795–798
DeVita MA, Braithwaite RS, Mahidhara R, Stuart S, Foraida M, Simmons RL (2004) Use of medical emergency team responses to reduce hospital cardiopulmonary arrests. Qual Saf Health Care 13:251–254
Salamonson Y, Kariyawasam A, van Heere B, O'Connor C (2001) The evolutionary process of Medical Emergency Team (MET) implementation: reduction in unanticipated ICU transfers. Resuscitation 49:135–141
Foraida MI, DeVita MA, Braithwaite RS, Stuart SA, Brooks MM, Simmons RL (2003) Improving the utilization of medical crisis teams (Condition C) at an urban tertiary care hospital. J Crit Care 18:87–94
Hartin J, Cook J, Phillips E, Singer M, Webb A, Adam SK (2002) Using algorithms in critical care outreach: UCLH Trust Patient Emergency Response Team (PERT) algorithm for fluid challenge. Care of the Critically Ill 18:158–159
Sugrue M, Seger M, Kerridge R, Sloane D, Deane S (1995) A prospective study of the performance of the trauma team leader. J Trauma 38:79–82
Dodek P, Herrick R, Phang PT (2000) Initial management of trauma by a trauma team: effect on timeliness of care in a teaching hospital. Am J Med Qual 15:3–8
Goldhill DR (2000) Medical emergency teams. Care of the Critically Ill 16:209–212
Goldhill DR, White SA, Sumner A (1999) Physiological values and procedures in the 24 h before ICU admission from the ward. Anaesthesia 54:529–534
Subbe CP, Davies RG, Williams E, Rutherford P, Gemmell L (2003) Effect of introducing the Modified Early Warning score on clinical outcomes, cardio-pulmonary arrests and intensive care utilisation in acute medical admissions. Anaesthesia 58:797–802
Odell M, Forster AL, Rudman K, Bass F (2002) The critical care outreach service and the early warning system on surgical wards. Nurs Crit Care 7:132–135
Carberry M (2002) Implementing the modified early warning system: our experiences. Nurs Crit Care 7:220–226
Day BA (2003) Early warning system scores and response times: an audit. Nurs Crit Care 8:156–164
Pittard AJ (2003) Out of our reach? Assessing the impact of introducing a critical care outreach service. Anaesthesia 58:882–885
Fox N, Rivers J (2001) Critical care outreach team sees fall in cardiac arrests. Nurs Times 97:34–35
Priestley G, Watson W, Rashidian A, Mozley C, Russell D, Wilson J, Cope J, Hart D, Kay D, Cowley K, Pateraki J (2004) Introducing Critical Care Outreach: a ward-randomised trial of phased introduction in a general hospital. Intensive Care Med 30:1398–1404
Sterling C, Barrrera Groba C (2002) An audit of a patient-at-risk trigger scoring system for identifying seriously ill ward patients. Nurs Crit Care 7:215–219
Welch J (2004) Using assessment to identify and prevent critical illness. Nursing Times Plus 96:2–4
Stenhouse C, Coates S, Tivey M, Allsop P, Parker T (2000) Prospective evaluation of a modified Early Warning Score to aid earlier detection of patients developing critical illness on a surgical ward. Br J Anaesth 84:663P
Sharpley JT, Holden JC (2004) Introducing an early warning scoring system in a district general hospital. Nurs Crit Care 9:98–103
Bell MB, Konrad D, Granath F, Ekbom A, Martling CR (2006) Prevalence and sensitivity of MET-criteria in a Scandinavian university hospital. Resuscitation 70:66–73
Andersson C, Olsson M, Hvarfner A, Engström M (2006) A survey among nurses regarding the effects of establishment of a medical emergency team [abstract]. Intensive Care Med 32(Suppl 1):S145
Van Delft TGM, Issa DE, Ten Kate RW, Van der Steen MS (2006) The medium care unit within developing intensive care: niche or needless? [abstract]. Intensive Care Med 32(Suppl 1):S165
Campello G, Martins M, Carvalho F, Dias C, Granja C (2005) Hospital emergency medical teams and massive basic life support – impact on mortality [abstract]. Intensive Care Med 31(Suppl 1):S12
Oggioni R, Bandini F, Fradella G, Pedullà A, Remorini L, Tonelli MV (2005) The in-hospital patients at risk: a multidisciplinary project of outreach team to improve the quality and continuity of care [abstract]. Intensive Care Med 31(Suppl 1):S130
Acknowledgements
This study was funded by the UK National Health Service Research & Development Service Delivery & Organisation programme (SDO/74/2004). We thank the 31 hospitals that provided data for this analysis: Alex Larkin, Royal Oldham Hospital; Carol Tune, Royal Shrewsbury Hospital; Chris Subbe, Wrexham Maelor Hospital; Clare Bamforth, Dewsbury & District Hospital; David Goldhill, Royal London Hospital; Elizabeth Hogbin, Norfolk & Norwich University Hospital; Jackie Hogan/Stephen Murray, North Manchester Hospital; Jane Chandler/Erin Povey, Wexham Park Hospital; Jane Saunders, Bradford Royal Infirmary; Jane Viner, Torbay Hospital; Kath Daly, Guy's and St. Thomas' Hospital; Kelly Henley, James Cook University Hospital; Lindsay Green/Samantha Fox, Good Hope Hospital; Lorna Johnson, Leeds General Infirmary and St. James's University Hospital; Louise Stock, University Hospital Lewisham; Mike Heap/Kate Bray, Northern General Hospital and Royal Hallamshire Hospital; Natasha Williamson/Claire Brown, Hinchingbrooke Hospital; Pat Eden & Lee Hubbard, Royal National Orthopaedic Hospital; Paul Seymour/David Watts, Bromley Hospital; Ruth Mullett/Karen Robins, Alexandra Hospital and Worcester Royal Hospital; Sally Smith, Kent & Sussex Hospital and Maidstone Hospital; Sarah Ingleby/Chris Booth, Manchester Royal Infirmary; Sheila Adam, University College London Hospitals; Valerie Forde, University Hospitals Coventry; Wendy Watson/Julie Southwell, York Hospital; Wendy Wharton/Peter Groom, Southampton General Hospital.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Gao, H., McDonnell, A., Harrison, D.A. et al. Systematic review and evaluation of physiological track and trigger warning systems for identifying at-risk patients on the ward. Intensive Care Med 33, 667–679 (2007). https://doi.org/10.1007/s00134-007-0532-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-007-0532-3