Abstract
Aims/hypothesis
Cytotoxic T cells and macrophages contribute to beta cell destruction in type 1 diabetes at least in part through the production of cytokines such as IL-1β, IFN-γ and TNF-α. We have recently shown the IL-17 pathway to be activated in circulating T cells and pancreatic islets of type 1 diabetes patients. Here, we studied whether IL-17A upregulates the production of chemokines by human pancreatic islets, thus contributing to the build-up of insulitis.
Methods
Human islets (from 18 donors), INS-1E cells and islets from wild-type and Stat1 knockout mice were studied. Dispersed islet cells were left untreated, or were treated with IL-17A alone or together with IL-1β+IFN-γ or TNF-α+IFN-γ. RNA interference was used to knock down signal transducer and activator of transcription 1 (STAT1). Chemokine expression was assessed by quantitative RT-PCR, ELISA and histology. Cell viability was evaluated with nuclear dyes.
Results
IL-17A augmented IL-1β+IFN-γ- and TNF-α+IFN-γ-induced chemokine mRNA and protein expression, and apoptosis in human islets. Beta cells were at least in part the source of chemokine production. Knockdown of STAT1 in human islets prevented cytokine- or IL-17A+cytokine-induced apoptosis and the expression of particular chemokines, e.g. chemokine (C-X-C motif) ligands 9 and 10. Similar observations were made in islets isolated from Stat1 knockout mice.
Conclusions/interpretation
Our findings indicate that IL-17A exacerbates proinflammatory chemokine expression and secretion by human islets exposed to cytokines. This suggests that IL-17A contributes to the pathogenesis of type 1 diabetes by two mechanisms, namely the exacerbation of beta cell apoptosis and increased local production of chemokines, thus potentially aggravating insulitis.
Similar content being viewed by others
Introduction
Pancreatic beta cells, like neurons and other non-immune cell types, have a vigorous cellular self-defence or cell-autonomous immunity, which contributes to defence against infections (mostly viral) and leads to local inflammation [1, 2]. Key steps of the beta cell-autonomous immune response are regulated by candidate genes for type 1 diabetes, including viral recognition, regulation of inflammation and cross-talk with the adaptive immune response [3–5]. Excessive pancreatic beta cell-autonomous immunity, in crosstalk with endoplasmic reticulum stress and other signals, exacerbates inflammation, beta cell apoptosis and the progression to type 1 diabetes [6]. The triggering of the full autoimmune response in type 1 diabetes probably depends on a ‘dialogue’ between beta cells and the immune system, regulated by the local release of chemokines and cytokines, which attract and stimulate cells of the immune system such as macrophages and cytotoxic T lymphocytes [7–9]. These cells contribute to selective beta cell destruction both directly and via the production of proinflammatory cytokines such as IL-1β, IFN-γ and TNF-α [2].
The production of chemokines by pancreatic islets takes place in response to ‘danger signals’, such as a viral infection, or following signals provided by dying cells, IL-1β+IFN-γ and TNF-α+IFN-γ [2, 7, 10]. Chemokine expression is also present in islets from type 1 diabetic patients, confirming the clinical relevance of these experimental observations [11].
As insulitis progresses, the nature of local invasion by immune cells probably changes, with an increased contribution by IL-17-secreting CD4+ T cells (T helper [Th] cell 17), together with Th1 cells. Th17 cells have been implicated in several autoimmune diseases, including multiple sclerosis, psoriasis, inflammatory bowel disease and rheumatoid arthritis. Th17 cells produce the cytokine IL-17A, which is also produced by dendritic cells, macrophages and natural killer cells [12–14]. Neutralisation of IL-17A prevents diabetes in NOD mice when started at 10 weeks of age [15]. Moreover, circulating IL-17+ and beta cell-specific autoreactive CD4+ cells are present in the circulation of type 1 diabetes patients at diagnosis [16, 17] and are associated with diabetes in NOD mice [18]. Importantly, increased expression of IL-17A was detected in the islets of a recently diagnosed type 1 diabetic patient [17] and increased numbers of Th17 cells are present in pancreatic lymph nodes of patients with long-term type 1 diabetes [19]. IL-17A enhances IL-1β+IFN-γ- or TNF-α+IFN-γ-induced apoptosis in rodent and human beta cells [16, 17, 20] and IL-1β+IFN-γ upregulates the expression of the beta cell IL-17A receptor via the transcription factors nuclear factor-κB (NF-κB) and signal transducer and activator of transcription (STAT) 1 [17]. This raises the possibility that beta cell apoptosis in type 1 diabetes is initially triggered by Th1 cells and macrophages through the production of IL-1β and IFN-γ, and by other mechanisms, these processes then being followed by the additional pro-apoptotic effects of IL-17A-secreting Th17 cells [17].
As discussed above, the early events leading to the build-up of insulitis involve local production of chemokines, raising the possibility that IL-17A, apart from contributing to beta cell apoptosis, also modulates the production and release of chemokines by islet cells. To test this hypothesis, we characterised the pattern of chemokine expression induced by IL-17A, alone or together with IL-1β, IFN-γ and TNF-α, in human and mouse islets of Langerhans. The data obtained suggest that IL-17A increases the expression and release of key chemokines, in part via activation of STAT1. They also suggest the intriguing possibility that IL-17A contributes to the pathogenesis of type 1 diabetes by two mechanisms, namely exacerbation of beta cell apoptosis and increased local production of chemokines, thus potentially aggravating insulitis.
Methods
Ethics statement
Human islet collection and handling were approved by the local Ethics Committee in Pisa, Italy.
Wild-type C57BL/6 mice were purchased from Harlan (Horst, the Netherlands). Stat1 knockout (KO) mice (on a C57BL/6 background) were a kind gift from David Levy (New York University, New York, NY, USA) [21]. All experimental procedures in mice were approved and performed in accordance with the Ethics Committees of the KU, Leuven, Belgium.
Culture of human islet cells, mouse islets, INS-1E cells and the human beta cell line EndoC-βH1
Human islets from 18 non-diabetic donors were isolated in Pisa using collagenase digestion and density gradient purification [22]. The donors (ten women, eight men) were 65 ± 3 years old and had a BMI of 26 ± 1 kg/m2 (mean ± SEM) (electronic supplementary material [ESM] Table 1). Beta cell purity, as evaluated by immunofluorescence for insulin (ESM Table 2), was 56% ± 4% (mean ± SEM). The islets were cultured in M199 culture medium containing 5.5 mmol/l glucose and sent to Brussels within 1 to 5 days after isolation, where they were dispersed and cultured in Ham’s F-10 medium containing 6.1 mmol/l glucose (Invitrogen Life Technologies, Paisley, UK) as described [3, 23].
Mouse islets were isolated from 2- to 3-week-old mice and cultured as previously described [21, 24]. After a 24 h recovery period, 150 islets per condition were exposed to cytokines in 1 ml medium.
The rat insulin-producing INS-1E cell line, kindly provided by C. Wollheim (University of Geneva, Switzerland) [25], was maintained in RPMI 1640 GlutaMAX-I medium (Invitrogen) [26].
The human beta cell line EndoC-βH1, kindly provided by R. Scharfmann (University of Paris, Paris, France) [27], was cultured in DMEM containing 5.6 mmol/l glucose, as described [27]. The same medium, but with 2% (vol./vol.) FBS was used for cytokine treatment.
Cell treatment
The following cytokine concentrations were used, based on previous dose–response experiments performed by our group [28–32]: (1) recombinant human IL-1β (R&D Systems, Abingdon, UK), 10 or 50 units/ml, respectively, for INS-1E cells or human islet cells, mouse islets and EndoC-βH1 cells; (2) recombinant rat IFN-γ (R&D Systems), 100 units/ml for INS-1E cells; (3) murine IFN-γ for mouse islets and human IFN-γ for human islet cells or EndoC-βH1 cells (Peprotech, London, UK), 1,000 units/ml; (4) recombinant murine TNF-α (Innogenetics, Gent, Belgium) for all cell types, 1,000 units/ml; and (5) recombinant murine IL-17A and human IL-17A (R&D Systems) for INS-1E cells and mouse islets, or for human islet cells and EndoC-βH1 cells, 20 ng/ml. Culture supernatant fractions were collected for chemokine measurements.
RNA interference
The short interfering RNAs (siRNA) used in the study are described in ESM Table 3. The optimal concentration of siRNAs used for cell transfection (30 nmol/l) was established previously [23, 33]. Cells were transfected using Lipofectamine RNAiMAX lipid reagent (Invitrogen) as previously described [33, 34]. Allstars negative control siRNA (Qiagen, Venlo, the Netherlands) was used as negative siRNA control (siCTRL). This siCTRL does not affect beta cell gene expression or insulin release, compared with non-transfected cells [33]. After 16 h of transfection, cells were cultured for a 48 h recovery period before exposure to cytokines.
Assessment of cell viability
The percentage of viable, apoptotic and necrotic cells was determined after 15 min incubation with DNA-binding dyes propidium iodide (5 μg/ml) (Sigma-Aldrich, Poole, UK) and Hoechst dye 33342 (5 μg/ml) (Sigma-Aldrich). Determination was by two independent researchers, one of whom was unaware of sample identity [35, 36]. A minimum of 600 cells were counted for each experimental condition. In some cases, apoptosis was also confirmed by caspase-3 cleavage.
mRNA extraction and real-time PCR
Poly(A)+ mRNA was isolated from human islet cells or EndoC-βH1 cells using a kit (Dynabeads mRNA Direct; Invitrogen) and reverse-transcribed as previously described [7, 36]. The real-time PCR amplification reaction was done using iQ SYBR Green Supermix on a LightCycler instrument (Roche-Diagnostic, Indianapolis, IN, USA) and the concentration of the gene of interest was calculated as copies per μl using the standard curve method [36, 37]. Gene expression values in human islets were corrected by the housekeeping gene β-actin, the expression of which is not modified in these cells by cytokine treatment [8, 23], and normalised to the highest value in each individual experiment. The primers used in this study are provided in ESM Table 4.
Total RNA from mouse islets was extracted using a kit (RNAeasy Micro; Qiagen). For cDNA synthesis, 100 ng was reverse-transcribed using Superscript II Reverse Transcriptase and oligo d(T)16 (Invitrogen). Real-time PCR was performed with Fast SYBR Green Master Mix and gene-specific primers or with TaqMan Fast Universal Master Mix and gene-specific primers plus TaqMan probe (Applied Biosystems-Life Technologies, Foster City, CA, USA) (ESM Table 4). PCR amplification was carried out in a real-time PCR system (StepOnePlus; Applied Biosystems). Relative RNA expression was calculated using the comparative cycle threshold method (Ct), after normalisation to the geometrical mean of three housekeeping genes (Rpl27, Hprt and β-actin).
Chemokine ELISA
The human chemokines, chemokine (C-X-C motif) ligand (CXCL) 1 (GRO-α), CXCL8 (IL-8), chemokine (C-C motif) ligand (CCL) 20 (MIP-3α), CCL2 (MCP-1), CXCL10 (IP-10), CXCL9 (MIG) and CCL5 (RANTES) were measured using an ELISA array kit (Custom Multi-Analyte kit; SABiosciences, Frederick, MD, USA) [3]. This is a semi-quantitative assay that does not include a standard curve. Absorbance at 450 nm was measured, corrected by readings at 570 nm and normalised to the geometric mean of β-actin mRNA expression to allow an approximate correction for the amount of islet cells in the preparations. Final results were expressed as arbitrary units. Release of the human chemokines CXCL1, CCL20, CXCL10 and CXCL9 was subsequently measured after STAT1 knockdown (KD) using quantitative ELISA kits (Quantikine; R&D Systems). Release of the mouse chemokines CXCL1 and CCL20 was measured in wild-type and Stat1 KO islets using a quantitative ELISA kit (Quantikine; R&D Systems) and data obtained were normalised to the number of islets.
Chemotaxis assay
C57BL/6 islets were cultured for 24 h as described above and the supernatant fractions collected. For the chemotaxis assay, thioglycolate-elicited peritoneal cells from 8-week-old C57BL/6 mice were seeded in the top chamber of transwell plates (106 cells per well; 5.0 μm) (Costar; Corning, Corning, NY, USA), while conditioned islet medium was added to the lower chambers of the transwell plates. Following an incubation time of 2 h at 37°C in 5% CO2, the cells in the lower chamber were collected and stained for CD45 using a directly conjugated antibody (eBioscience, San Diego, CA, USA). To minimise non-specific binding, the cells were first pre-incubated with anti-CD16/CD32 (clone 2.4G2; eBiosience). Dead cells were excluded by using Fixable Live/Dead Yellow stain (Invitrogen). The absolute number of migrated leucocytes was determined by CountBright absolute counting beads (Invitrogen) according to the manufacturer’s specifications. Data acquisition was performed on a Gallios™ flow cytometer (Beckman Coulter, Brea, CA, USA) and Kaluza™ software (Beckman Coulter) was used for data analysis.
Western blot
Cells were washed with cold PBS and lysed using Laemmli buffer. Total protein was extracted and resolved by 8–10% SDS-PAGE, transferred to a nitrocellulose membrane and immunoblotted with the specific antibodies for the protein of interest (ESM Table 2) as described [23]. The intensity values for the proteins were corrected by the values of the housekeeping protein α-tubulin.
Promoter reporter assays
INS-1E cells were co-transfected using Lipofectamine 2000 (Invitrogen) with the internal control pRL-CMV encoding Renilla luciferase (Promega, Madison, WI, USA) and a luciferase promoter–reporter construct containing five NF-κB consensus binding sites (pNF-κB-Luciferase) (BD Biosciences, Palo Alto, CA, USA). Luciferase activities were assayed after 4 h of cytokine treatment.
Histology
Whole human islets were collected, fixed in formaldehyde and paraffin-embedded. Sections (5 μm thick) were subjected to antigen retrieval by boiling in 10 mmol/l citrate buffer (pH 6) for 10 min. After overnight incubation at 4°C and with primary antibodies against the chemokines CXCL1, CXCL10 and CCL20, slides were double-stained for 1 h and at room temperature with an anti-insulin or an anti-glucagon antibody. Alexa Fluor fluorescent secondary antibodies (Molecular Probes-Invitrogen, Leiden, the Netherlands) were applied for 1 h at room temperature (ESM Table 2). Immunofluorescence was visualised on a Zeiss microscope equipped with a camera (Zeiss-Vision, Munich, Germany), as previously described [38].
Statistical analysis
Data are expressed as mean ± SEM. A significant difference between experimental conditions was assessed by ANOVA followed by a paired Student’s t test with Bonferroni’s correction. A value of p < 0.05 was considered statistically significant.
Results
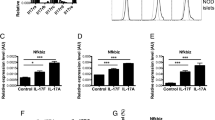
IL-17A exacerbates cytokine-induced apoptosis in rat INS-1E cells, mouse islet cells and in the human beta cell line EndoC-βH1
We have previously shown that IL-17A augments apoptosis in human islet cells or rat beta cells exposed to IL-1β+IFN-γ or TNF-α+INF-γ [17]. Here, these findings were expanded by the observations that IL-17A increased apoptosis induced by IL-1β+IFN-γ in rat INS-1E cells, as judged by nuclear dyes and caspase 3 cleavage (Fig. 1a–c); IL-17A also augmented cell death in mouse islet cells exposed to IL-1β+IFN-γ or TNF-α+INF-γ, this effect being particularly marked in the case of TNF-α+INF-γ, with an IL-17A-induced 84-fold increase in cell death compared with TNF-α+INF-γ alone (Fig. 1d). On the other hand, IL-17A alone at 20 ng/ml did not increase beta cell death (Fig. 1d). The effects of IL-17A were further investigated in a dose–response experiment in mouse pancreatic islets (experimental conditions as in Fig. 1d). The percentage of apoptotic cells was 2.3% ± 0.6%, 3.2% ± 0.6% and 1.8% ± 0.2% after exposure to 20, 100 and 200 ng/ml IL-17A, respectively, a finding that was similar to the percentage of apoptotic cells observed in control (non-cytokine treated) islet cells, i.e. 3.5% ± 1.2% (n = 4) (data not shown). These observations confirm that IL-17A alone, even at high concentrations, is not toxic to primary beta cells. We also observed that IL-1β+IFN-γ induced apoptosis in human EndoC-βH1 cells and that this was mildly increased by IL-17A (Fig. 1e). To our knowledge this is the first evidence that this human cell line is sensitive to cytokine-induced apoptosis.
IL-17A exacerbates cytokine-induced apoptosis in INS-1E cells, mouse islets and the human beta cell line EndoC-βH1. (a–c) INS-1E cells, (d) mouse islet cells and (e) human EndoC-βH1 cells were left untreated, or were treated with IL-17A alone and with combinations of IL-1β+IFN-γ or TNF-α+IFN-γ with or without IL-17A, as indicated. INS-1E (a), mouse islet cells (d) and human beta cell line EndoC-βH1 apoptosis (e) were evaluated after 24, 96 and 48 h treatment, respectively. (b) Cleaved caspase-3 and α-tubulin protein abundance were evaluated by western blot. Optical density quantification (c) of cleaved caspase-3 was corrected by α-tubulin. Results are mean ± SEM of four, six and eight independent experiments for INS-1E cells, mouse islets and the human beta cell line EndoC-βH1, respectively; ***p < 0.001 vs untreated cells and ††† p < 0.001 as indicated; analysis was by ANOVA followed by paired Student’s t test with Bonferroni’s correction
IL-17A exacerbates cytokine-induced chemokine expression by human and mouse islets
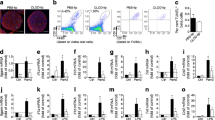
IL-17A induced a mild increase in the expression and release of CXCL1, CXCL8 and CCL20, and augmented chemokine expression and release induced by TNF-α+INF-γ (and also in most cases by IL-1β+IFN-γ) in human islets (Fig. 2a–f). The stimulatory effects of IL-17A were less marked in the case of CCL2 and CXCL10 (Fig. 2g–j), and were not present for CCL5 and CXCL9 (data not shown). Similar to these observations, IL-17A also exacerbated cytokine-induced CXCL1 and CCL20 expression in mouse islets (ESM Fig. 1a–c). When added to TNF-α+INF-γ, IL-17A also augmented the chemotaxis of live CD45+ leucocytes (ESM Fig. 1d).
IL-17A exacerbates cytokine-induced mRNA expression and protein secretion of chemokines by human islets. Dispersed human islets were left untreated, or were treated for 48 h with IL-17A alone or with combinations of IL-1β+IFN-γ or TNF-α+IFN-γ, with or without IL-17A as indicated. CXCL1 (a), CXCL8 (c), CCL20 (e), CCL2 (g) and CXCL10 (i) mRNA expression was assayed by RT-PCR and normalised to the housekeeping gene β-actin. Secreted CXCL1 (b), CXCL8 (d), CCL20 (f), CCL2 (h) and CXCL10 (j) were quantified in the cultured medium by ELISA and are expressed as arbitrary units (AU). Results are mean ± SEM of five to eight independent experiments in human islets; *p < 0.05, **p < 0.01 and ***p < 0.001 vs untreated cells; † p < 0.05, †† p < 0.01 and ††† p < 0.001 as indicated by bars; analysis was by ANOVA followed by paired Student’s t test with Bonferroni’s correction
Immunohistochemistry indicated detectable protein expression of CXCL1, CCL20 and CXCL10 in islets exposed to IL-1β+IFN-γ and TNF-α+INF-γ, with or without IL-17A, but not in control islets or islets treated with IL-17A alone (data not shown). Double immunofluorescence confirmed that beta cells were at least in part the source of chemokine production after cytokine treatment, as shown by the presence of cells double-positive for insulin and CXCL1, CCL20 or CXCL10 (Fig. 3c, f, i). Interestingly, while expression of CXCL1 and CXCL10 was diffuse in the cytosol, CCL20 staining was more ‘punctiform’ in appearance. The percentage of cells double-positive for insulin and chemokines was 60% for CXCL1, 35% for CCL20 and 70% for CXCL10, indicating that not all beta cells expressed the chemokines.
Beta cells are at least in part responsible for the production of chemokines. Human islets were left untreated, or were treated for 48 h with IL-17A alone or with combinations of IL-1β+IFN-γ or TNF-α+IFN-γ, with or without IL-17A. After the treatment, islets were collected and paraffin-embedded. Fluorescent microscopy analysis of (a, d, g) insulin (INS, red), (b) CXCL1, (e) CCL20 and (h) CXCL10 (all green) shows the presence of cells double-positive for insulin and (c) CXCL1 (merged panels, yellow), (f) CCL20 (merged panels, yellow) and (i) CXCL10 (merged panels, yellow). Nuclear staining was performed with Hoechst dye. Images are representative of IL-1β+IFN-γ+IL-17A-treated islets of two independent human islet preparations. Similar observations were made in TNF-α+IFN-γ+IL-17A-treated islets (data not shown)
IL-17A induces an early increase in IL-1β + IFN-γ-induced STAT1 phosphorylation and interferon regulatory factor 1 activation in INS-1E cells
It has been previously shown in other cell types that IL-17A activates the transcription factor, NF-κB [39, 40]. NF-κB is important for chemokine expression and cell apoptosis in beta cells exposed to IL-1β+IFN-γ [2, 28]. The addition of IL-17A, however, neither increased IκBα degradation (an early step in NF-κB activation) (ESM Fig. 2a, b), nor augmented IL-1β+IFN-γ-induced activation of a luciferase reporter containing several NF-κB binding sites (ESM Fig. 2c), arguing against a role for NF-κB as a mediator of IL-17A effects in beta cells.
STAT1 is another master regulator of cytokine-induced pancreatic beta cell apoptosis and chemokine production [21, 41]. The addition of IL-17A to IL-1β+IFN-γ increased STAT1 phosphorylation and interferon regulatory factor 1 (IRF-1) (a downstream STAT1 transcription factor) expression at earlier time points (2–6 h) (Fig. 4a–c), which led us to perform additional experiments where IL-17A and other cytokines were added to beta cells with blocked STAT1 signalling. Since STAT1, but not IRF-1, upregulate chemokine expression [41], these experiments focused on STAT1.
IL-17A induces an early increase in IL-1β+IFN-γ-induced STAT1 phosphorylation and IRF-1 activation in INS-1E cells. (a) Phosphorylated (p-)STAT1, IRF-1 and α-tubulin proteins were evaluated by western blot. (b) Optical density quantification of p-STAT1 and (c) IRF-1 was corrected by α-tubulin. INS-1E cells were left untreated (white bars), or treated with IL-17A (light grey bars) and a combination of IL-1β+IFN-γ with (black bars) or without (dark grey bars) IL-17A for times as indicated. Results are mean ± SEM of seven independent experiments; *p < 0.05, **p < 0.01 and ***p < 0.001 vs untreated cells for each time point, and † p < 0.05 and ††† p < 0.001 as indicated; analysis was by ANOVA followed by paired Student’s t test with Bonferroni’s correction
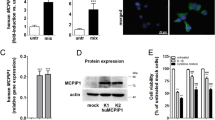
STAT1 KD or KO decreases cytokine-induced apoptosis and the production of specific chemokines in INS-1E cells, and in human and mouse islets
STAT1 KD in INS-1E cells (ESM Fig. 3a, b) significantly decreased cell death after IL-1β+IFN-γ and TNF-α+IFN-γ treatment, with or without the addition of IL-17A (Fig. 5a). The percentage of inhibition of cell death achieved by the blockade of STAT1 in INS-1E cells (Fig. 5a) was similar in the presence or absence of IL-17A, suggesting that inhibition of this transcription factor mainly prevents the pro-apoptotic signalling provided by IFN-γ [21, 41]. To decrease STAT1 expression in dispersed human islets, we tested three different siRNAs in HeLa cells (ESM Table 3). The best results were obtained with siSTAT1#2 (ESM Fig. 3c, d), with which all subsequent experiments were performed. An 80% and 60% decrease of STAT1 and IRF1 mRNA expression, respectively, was observed in human islets transfected with STAT1 siRNA and subsequently treated with IL-1β+IFN-γ or TNF-α+IFN-γ, with or without the addition of IL-17A (ESM Fig. 3e, f). STAT1 KD reduced apoptosis to the same extent in dispersed human islet cells exposed to IL-1β+IFN-γ and IL-1β+IFN-γ+IL-17A (Fig. 5b). There was also a non-significant trend for a decrease in apoptosis following treatment with TNF-α+IFN-γ with or without IL-17A (Fig. 5b).
STAT1 KD or KO decreases cytokine-induced apoptosis in INS-1E cells, dispersed human islets and mouse islets. (a) INS-1E cells and (b) dispersed human islets were transfected with siCTRL (white bars) or with siSTAT1 (black bars). After 48 h of recovery, cells were left untreated, or were treated with IL-17A alone or with combinations of IL-1β+IFN-γ or TNF-α+IFN-γ, with or without IL-17A. INS-1E (a) and human islets cell apoptosis (b) was evaluated after 24 and 48 h of treatment, respectively. (c) Mouse islets of wild-type (C57Bl/6) (white bars) and Stat1 KO (black bars) mice were left untreated, or were treated as above (a, b) and cell apoptosis evaluated after 48 h and (d) 96 h. Results are mean ± SEM of three, five and nine independent experiments for INS-1E cells, mouse islets or human islets, respectively; *p < 0.05, **p < 0.01 and ***p < 0.001 vs untreated cells; † p < 0.05, †† p < 0.01 and ††† p < 0.001 as indicated; ‡ p < 0.05, ‡‡ p < 0.01 and ‡‡‡ p < 0.001 as indicated; analysis was by ANOVA followed by paired Student’s t test with Bonferroni’s correction
We next examined the effects of STAT1 deletion by using islets isolated from Stat1 KO mice. After 48 or 96 h of IL-1β+IFN-γ or TNF-α+IFN-γ treatment, with or without addition of IL-17A, protection against cytokine-induced apoptosis was complete (Fig. 5c, d), confirming here that, as shown elsewhere [21], STAT1 is a crucial mediator of beta cell apoptosis induced by cytokine mixtures containing IFN-γ.
Blocking the STAT1 pathway in human islets reduced the expression of CXCL9 and CXCL10 at the mRNA (Fig. 6a, c) and protein levels (Fig. 6b, d), and also decreased mRNA expression of these chemokines in mouse islets (Fig. 6e, f). siSTAT1 also decreased CXCL8 (also known as IL8) mRNA expression in human islets exposed to TNF-α+IFN-γ+IL-17A (ESM Fig. 4a). On the other hand, CXCL1 and CCL20, the chemokines most upregulated by IL-17A, (Figs 2 and 7), showed in most cases a paradoxical increase in expression following inhibition of STAT1 in human islets (Fig. 7a–d). This was confirmed in islets isolated from Stat1 KO mice, where the absence of STAT1 led to a major increase in CXCL1 expression (Fig. 7e, f) and a less marked increase in CCL20 in the presence of IL-1β+IFN-γ+IL-17A, TNF-α+IFN-γ or TNF-α+IFN-γ+IL17A (protein level only) (Fig. 7g, h). We have previously shown that IRF-7 plays an important role in the regulation of chemokine expression by TNF-α+IFN-γ [42], but this does not explain the present observations, as STAT1 KD decreased IRF-7 expression (ESM Fig. 4b). Taken together, these findings indicate a complex regulation of chemokine expression in human and mouse pancreatic islets, which may contribute to different ‘chemokine signatures’ depending on the proinflammatory stimulus delivered to the islet cells and the downstream transcription factors activated.
STAT1 KD or KO decreases cytokine-induced mRNA expression and protein secretion of CXCL10 and CXCL9 by human and mouse islets. (a–d) Dispersed human islets were transfected with siCTRL (white bars) or with siSTAT1 (black bars). After 48 h of recovery, cells were left untreated, or were treated for 48 h with IL-17A alone, or with combinations of IL-1β+IFN-γ or TNF-α+IFN-γ, with or without IL-17A. CXCL10 (a) and CXCL9 (c) mRNA expression was assayed by RT-PCR and normalised to the housekeeping gene β-actin. (b) Secreted CXCL10 and (d) CXCL9 were quantified in the cultured medium by ELISA and expressed in arbitrary units (AU) as fold-change compared with untreated cells. (e–f ) Mouse islets of wild-type (white bars) and Stat1 KO (black bars) mice were left untreated or treated as above (a–d). Cxcl10 (e) and Cxcl9 (f ) mRNA expression was assayed by RT-PCR and normalised to three different housekeeping (hk) genes (β-actin, Rpl27 and Hprt). Results are mean ± SEM of three and six independent experiments for mouse islets or human islets, respectively; ***p < 0.001 vs untreated cells; † p < 0.05, †† p < 0.01 and ††† p < 0.001 as indicated; ‡‡‡ p < 0.001 as indicated; analysis was by ANOVA followed by paired Student’s t test with Bonferroni’s correction
STAT1 KD or KO causes a paradoxical increase in cytokine-induced mRNA expression and protein secretion of CXCL1 and CCL20 by human and mouse islets. (a–d) Dispersed human islets were transfected with siCTRL (white bars) or with siSTAT1 (black bars). After 48 h of recovery, cells were left untreated, or were treated for 48 h with IL-17A alone, or with combinations of IL-1β+IFN-γ or TNF-α+IFN-γ, with or without IL-17A. (a) CXCL1 and (c) CCL20 mRNA expression was assayed by RT-PCR and normalised to the housekeeping gene β-actin. (b) Secreted CXCL1 and (d) CCL20 were quantified in the cultured medium by ELISA and expressed in arbitrary units (AU) as fold change compared with untreated cells. (e–h) Mouse islets of wild-type (white bars) and Stat1 KO (black bars) mice were left untreated or treated as above (a–d). (e) Cxcl1 and (g) Ccl20 mRNA expression was assayed by RT-PCR and normalised to three different housekeeping (hk) genes (β-actin, Rpl27 and Hprt). (f) Secreted CXCL1 and (h) and CCL20 were quantified in the culture medium by ELISA. Results are mean ± SEM of three and six independent experiments for mouse or human islets, respectively; *p < 0.05, **p < 0.01 and ***p < 0.001 vs untreated cells; † p < 0.05, †† p < 0.01 and ††† p < 0.001 as indicated; ‡ p < 0.05, ‡‡ p < 0.01 and ‡‡‡ p < 0.001 as indicated; analysis was by ANOVA followed by paired Student’s t test with Bonferroni’s correction
Discussion
During the progression of insulitis in type 1 diabetes, pancreatic beta cells are probably exposed to changing groups of cytokines. Recent studies indicate an increased presence of Th17 cells among circulating T cells and in the pancreatic islets of type 1 diabetes patients [16–19]. Th17 cells secrete the cytokine IL-17A, which we and others have shown to exacerbate IL-1β+IFN-γ- and TNF-α+IFN-γ-induced apoptosis in MIN6 cells and human islets [16, 17]. In the present study, we confirmed that IL-17A augments pro-apoptotic signalling by IL-1β+IFN-γ and TNF-α+IFN-γ in mouse islets, rat INS-1E cells and the human insulin-producing cell line EndoC-βH1, extending our previous observations in human islets and rat beta cells [17]. The use here of a large number of independent human islet preparations, mouse islets and clonal cell lines is particularly important, as it allows solid conclusions regarding the deleterious effects of IL-17A in the context of isolated/clonal beta cells and beta cells studied together with other islet cells. However, a limitation of the present study is its purely in vitro nature, with the isolated islets or beta cells studied being separated from the immune system. It will therefore be important to confirm the present findings in in vivo models or, preferably, in pancreatic or islet samples from type 1 diabetes patients.
Apart from inducing cell death, cytokines also trigger the release of chemokines by pancreatic islets, contributing to the attraction and activation of more immune cells [2, 10, 11]. The chemokine ‘signature’ of pancreatic islets seems to vary depending on the cytokine stimulus provided. For instance, the pattern of chemokines induced by IL-1β+IFN-γ in pancreatic beta cells is markedly different from that induced by TNF-α+IFN-γ [42]. We showed here that IL-17A alone induces a mild increase in expression of CXCL1, CXCL8, CCL2 and CCL20, in addition to its stimulatory effect on human islets exposed to IL-1β+IFN-γ and TNF-α+IFN-γ. These additive effects are most marked in the case of TNF-α+IFN-γ, affecting, preferentially, the chemokines CXCL1, CXCL8, CXCL10 and CCL20. A similar pattern of chemokine induction was observed in mouse islets. Immunofluorescence studies, including co-localisation with insulin, confirmed that human beta cells are, at least in part, the source of CXCL1, CCL20 and CXCL10 production in human islets.
Chemokines play an important role in the recruitment of leucocytes, monocytes and activated T cells to areas of inflammation in autoimmune diseases [43, 44]. CXCL1 is important in the recruitment of neutrophils, which infiltrate the pancreas and are present in low numbers in the periphery in diabetic patients [45]. CCL2 expression is increased in the islets of prediabetic female NOD mice [7], and transgenic overexpression of CCL2 in beta cells stimulates insulitis and progression to diabetes in mice with a B6D2 background [46]. On the other hand, isolated transgenic expression of CCL2 in NOD mouse beta cells decreases autoimmune-mediated beta cell destruction via induction of tolerogenic dendritic cells, suggesting a context-dependent effect of these proteins [47]. NOD mouse islets also show increased expression of CCL20 and CXCL10, with a peak at around 10 to 12 weeks of life, which precedes the outbreak of diabetes by 2 to 4 weeks. CCL20 is the only ligand for CCR6, which is expressed in Th17 and regulatory T cells [10, 48]. CXCL10 and CXCL9 are present in pancreatic islets from type 1 diabetic patients [11, 48], while infiltrating lymphocytes express the CXCL10 receptor, C-X-C chemokine receptor type 3 [11], suggesting a ‘dialogue’ between chemokine-producing islet cells and the invading immune cells. In NOD mice, the severity of insulitis is reduced in IL-17- or IL-17/IFN-γ receptor-deficient animals [49], indicating the importance of IL-17A in the attraction of immune cells. As suggested by the present data, this may be mediated via increased production of chemokines.
The mechanisms behind the proinflammatory and pro-apoptotic effects of IL-17A in beta cells remain to be determined. Our present findings rule out a major role for NF-κB and suggest that STAT1 mainly mediates the effects of IFN-γ, but not those of IL-17A. Indeed, KD or KO of STAT1 in human or rodent beta cells prevents apoptosis and the expression of CXCL9 and CXCL10 to the same extent in cells exposed to IL-1β+IFN-γ and TNF-α+IFN-γ, with or without IL-17A. On the other hand, STAT1 seems to have an inhibitory effect on expression levels of the chemokines CXCL1 and CCL20. This may contribute to the generation of specific ‘chemokine signatures’ depending on the original stimuli and downstream transcription factors activated. It has been shown that IL-17A modulates inflammation in lupus or rheumatoid arthritis via downregulation of the microRNA miR-23b [50], but we were not able to confirm this finding in our model (data not shown).
In conclusion, the present findings suggest that IL-17A contributes to the expression and secretion of chemokines by human and rodent islet cells, but the ultimate mechanisms behind the effects of IL-17A in beta cells remain to be clarified. Our observations indicate that IL-17A contributes to the pathogenesis of type 1 diabetes by two mechanisms, namely the exacerbation of beta cell apoptosis and increased local production of chemokines, thus potentially aggravating insulitis.
Abbreviations
- CCL:
-
Chemokine (C-C motif) ligand
- CXCL:
-
Chemokine (C-X-C motif) ligand
- IRF-1:
-
Interferon regulatory factor 1
- KD:
-
Knockdown
- KO:
-
Knockout
- NF-κB:
-
Nuclear factor-κB
- siCTRL:
-
Negative siRNA control
- siRNA:
-
Short interfering RNA
- STAT:
-
Signal transducer and activator of transcription
- Th:
-
T helper
References
Randow F, MacMicking JD, James LC (2013) Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 340:701–706
Eizirik DL, Colli ML, Ortis F (2009) The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat Rev Endocrinol 5:219–226
Eizirik DL, Sammeth M, Bouckenooghe T et al (2012) The human pancreatic islet transcriptome: expression of candidate genes for type 1 diabetes and the impact of pro-inflammatory cytokines. PLoS Genet 8:e1002552
Bergholdt R, Brorsson C, Palleja A et al (2012) Identification of novel type 1 diabetes candidate genes by integrating genome-wide association data, protein-protein interactions, and human pancreatic islet gene expression. Diabetes 61:954–962
Santin I, Eizirik DL (2013) Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and β-cell apoptosis. Diabetes Obes Metab 3:71–81
Eizirik DL, Miani M, Cardozo AK (2013) Signalling danger: endoplasmic reticulum stress and the unfolded protein response in pancreatic islet inflammation. Diabetologia 56:234–241
Chen MC, Proost P, Gysemans C, Mathieu C, Eizirik DL (2001) Monocyte chemoattractant protein-1 is expressed in pancreatic islets from prediabetic NOD mice and in interleukin-1β-exposed human and rat islet cells. Diabetologia 44:325–332
Cardozo AK, Kruhøffer M, Leeman R, Orntoft T, Eizirik DL (2001) Identification of novel cytokine-induced genes in pancreatic β-cells by high-density oligonucleotide arrays. Diabetes 50:909–920
Roep BO, Peakman M (2011) Diabetogenic T lymphocytes in human type 1 diabetes. Curr Opin Immunol 23:746–753
Cardozo AK, Proost P, Gysemans C, Chen MC, Mathieu C, Eizirik DL (2003) IL-1β and IFN-γ induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice. Diabetologia 46:255–266
Roep BO, Kleijwegt FS, van Halteren AG et al (2010) Islet inflammation and CXCL10 in recent-onset type 1 diabetes. Clin Exp Immunol 159:338–343
Bluestone JA, Herold K, Eisenbarth G (2012) Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature 464:1293–1300
Crome SQ, Wang AY, Levings MK (2010) Translational mini-review series on Th17 cells: function and regulation of human T helper 17 cells in health and disease. Clin Exp Immunol 159:109–119
Patel DD, Lee DM, Kolbinger F, Antoni C (2013) Effect of IL-17A blockade with secukinumab in autoimmune diseases. Ann Rheum Dis 72(Suppl 2):ii116–ii123
Emamaullee JA, Davis J, Merani S et al (2009) Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes 58:1302–1311
Honkanen J, Nieminen JK, Gao R et al (2010) IL-17 immunity in human type 1 diabetes. J Immunol 185:1959–1967
Arif S, Moore F, Marks K et al (2011) Peripheral and islet interleukin-17 pathway activation characterizes human autoimmune diabetes and promotes cytokine-mediated β-cell death. Diabetes 60:2112–2119
Martin-Orozco N, Chung Y, Chang SH, Wang YH, Dong C (2009) Th17 cells promote pancreatic inflammation but only induce diabetes efficiently in lymphopenic hosts after conversion into Th1 cells. Eur J Immunol 39:216–224
Ferraro A, Socci C, Stabilini A et al (2011) Expansion of Th17 cells and functional defects in T regulatory cells are key features of the pancreatic lymph nodes in patients with type 1 diabetes. Diabetes 60:2903–2913
Miljkovic D, Cvetkovic I, Momcilovic M, Maksimovic-Ivanic D, Stosic-Grujicic S, Trajkovic V (2005) Interleukin-17 stimulates inducible nitric oxide synthase-dependent toxicity in mouse β-cells. Cell Mol Life Sci 62:2658–2668
Gysemans CA, Ladrière L, Callewaert H et al (2005) Disruption of the γ-interferon signaling pathway at the level of signal transducer and activator of transcription-1 prevents immune destruction of β-cells. Diabetes 54:2396–2403
Lupi R, Dotta F, Marselli L et al (2002) Prolonged exposure to free fatty acids has cytostatic and pro-apoptotic effects on human pancreatic islets: evidence that β-cell death is caspase mediated, partially dependent on ceramide pathway, and Bcl-2 regulated. Diabetes 51:1437–1442
Moore F, Colli ML, Cnop M et al (2009) PTPN2, a candidate gene for type 1 diabetes, modulates interferon-γ-induced pancreatic β-cell apoptosis. Diabetes 58:1283–1291
Gysemans C, Callewaert H, Moore F et al (2009) Interferon regulatory factor-1 is a key transcription factor in murine β-cells under immune attack. Diabetologia 52:2374–2384
Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB (1992) Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology 130:167–178
Nogueira TC, Paula FM, Villate O et al (2013) GLIS3, a susceptibility gene for type 1 and type 2 diabetes, modulates pancreatic β-cell apoptosis via regulation of a splice variant of the BH3-Only protein Bim. PLoS Genet 9:e1003532
Ravassard P, Hazhouz Y, Pechberty S et al (2011) A genetically engineered human pancreatic β-cell line exhibiting glucose-inducible insulin secretion. J Clin Invest 121:3589–3597
Eizirik DL, Mandrup-Poulsen T (2001) A choice of death -the signal-transduction of immune-mediated β-cell apoptosis. Diabetologia 44:2115–2133
Kutlu B, Cardozo AK, Darville MI et al (2003) Discovery of gene networks regulating cytokine-induced dysfunction and apoptosis in insulin-producing INS-1 cells. Diabetes 52:2701–2719
Ortis F, Cardozo AK, Crispim D, Störling J, Mandrup-Poulsen T, Eizirik DL (2006) Cytokine-induced proapoptotic gene expression in insulin-producing cells is related to rapid, sustained, and nonoscillatory nuclear factor-kappaB activation. Mol Endocrinol 20:1867–1879
Eizirik DL, Pipeleers DG, Ling Z, Welsh N, Hellerström C, Andersson A (1994) Major species differences between humans and rodents in the susceptibility to pancreatic β-cell injury. Proc Natl Acad Sci U S A 91:9253–9256
Eizirik DL, Sandler S, Welsh N et al (1994) Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest 93:1968–1974
Moore F, Cunha DA, Mulder H, Eizirik DL (2012) Use of RNA interference to investigate cytokine signal transduction in pancreatic β-cells. Methods Mol Biol 820:179–194
Moore F, Santin I, Nogueira TC et al (2012) The transcription factor C/EBP delta has anti-apoptotic and anti-inflammatory roles in pancreatic β-cells. PLoS One 7:e31062
Hoorens A, van de Casteele M, Klöppel G, Pipeleers D (1999) Glucose promotes survival of rat pancreatic β-cells by activating synthesis of proteins which suppress a constitutive apoptotic program. J Clin Invest 98:1568–1574
Rasschaert J, Ladrière L, Urbain M et al (2005) Toll-like receptor 3 and STAT-1 contribute to double-stranded RNA + interferon-γ-induced apoptosis in primary pancreatic β-cells. J Biol Chem 280:33984–33991
Overbergh L, Valckx D, Waer M, Mathieu C (1999) Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine 11:305–312
Marhfour I, Lopez XM, Lefkaditis D et al (2012) Expression of endoplasmic reticulum stress markers in the islets of patients with type 1 diabetes. Diabetologia 55:2417–2420
Shalom-Barak T, Quach J, Lotz M (1998) Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-κB. Biol Chem 273:27467–27473
Schwandner R, Yamaguchi K, Cao Z (2000) Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J Exp Med 191:1233–1240
Moore F, Naamane N, Colli ML et al (2011) STAT1 is a master regulator of pancreatic β-cell apoptosis and islet inflammation. J Biol Chem 286:929–941
Ortis F, Naamane N, Flamez D et al (2010) Cytokines interleukin-1β and tumor necrosis factor-α regulate different transcriptional and alternative splicing networks in primary β-cells. Diabetes 59:358–374
Charo IF, Ransohoff RM (2006) The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med 354:610–621
Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P (2007) Role of chemokines in endocrine autoimmune diseases. Endocr Rev 28:492–520
Valle A, Giamporcaro GM, Scavini M et al (2013) Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes 62:2072–2077
Martin AP, Rankin S, Pitchford S, Charo IF, Furtado GC, Lira SA (2008) Increased expression of CCL2 in insulin-producing cells of transgenic mice promotes mobilization of myeloid cells from the bone marrow, marked insulitis, and diabetes. Diabetes 57:3025–3033
Kriegel MA, Rathinam C, Flavell RA (2012) Pancreatic islet expression of chemokine CCL2 suppresses autoimmune diabetes via tolerogenic CD11c + CD11b + dendritic cells. Proc Natl Acad Sci U S A 109:3457–3462
Sarkar SA, Lee CE, Victorino F et al (2012) Expression and regulation of chemokines in murine and human type 1 diabetes. Diabetes 61:436–446
Kuriya G, Uchida T, Akazawa S et al (2013) Double deficiency in IL-17 and IFN-γ signalling significantly suppresses the development of diabetes in the NOD mouse. Diabetologia 56:1773–1780
Zhu S, Pan W, Song X et al (2012) The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-α. Nat Med 18:1077–1086
Acknowledgements
We thank M. Igoillo-Esteve, Laboratory of Experimental Medicine-Université Libre de Bruxelles (ULB), for fruitful discussions and help with the analysis of human chemokines by ELISA. We also thank L. Ladriere, ULB, for help with the dispersion of human islets. We are grateful to the personnel from the ULB including I. Millard, A. Musuaya, M. Pangerl and S. Mertens. Thanks also to D. Lambrechts from the Laboratory of Clinical and Experimental Endocrinology (KU Leuven) for excellent technical support.
Funding
This work was supported by grants from the Fonds National de la Recherche Scientifique (FNRS) Belgium, the Communauté Française de Belgique-Action de Rechercher Concertéés (ARC), the European Union (projects NAIMIT and BetaBat, in the Framework Program 7 of the European Community) and the KU Leuven (Geconcerteerde Onderzoeksactie 2009/10 and 12/24). F.A. Grieco is a recipient of a postdoctoral fellowship from NAIMIT and was supported by a 1 year postdoctoral fellowship from the University of Siena and from Società Italiana di Diabetologia (Fo.Ri.SID) ONLUS, Italy.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
DLE, FAG, FM, FD, PM and CM contributed to the study concept and design. FAG, FM, FV, IS, OV, LM, DR, HK and LO acquired the data. DLE, LO and CM supervised the study. FAG and DLE drafted the manuscript. All authors revised the article and approved the final version to be published.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Fig 1
IL-17A exacerbates cytokine-induced mRNA expression and protein secretion of chemokines by mouse islets and chemotakis of CD45+ leukocytes. Mouse islets were left untreated or treated with IL-17A, combinations of IL-1β+IFN-γ or TNF-α+IFN-γ with or without IL-17A, for 48h (a-c) as indicated. CXCL1 (a) and CCL20 (c) mRNA expression was assayed by RT-PCR and normalized for three different housekeeping genes (β-actin, Rpl27 and Hprt). Secreted CXCL1 (b) was quantified in the culture medium by ELISA. Islet supernatants cultured for 24h in the presence of either vehicle, IL-17A, TNFα+IFN-γ or TNF-α+IFN-γ+IL-17A (d) were tested for their functional capacity to recruit leukocytes in a transwell assay. Thioglycolate-elicited peritoneal cells served as the source of total leukocytes. Results shown represent the relative increase in live CD45+ leukocytes recruited to the lower chamber as compared to the control condition. Results are mean ± SEM of 3-8 independent experiments. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs untreated cells; † p < 0.05, †† p < 0.01 and ††† p < 0.001 as indicated; ANOVA followed by paired Student’s t-test with Bonferroni’s correction. (PDF 89 kb)
ESM Fig 2
IL-17A does not affect IκB-α degradation or NF-κB activation. INS-1E cells were left untreated (white columns) or treated with IL-17A (light-gray columns), combinations of IL-1β+IFN-γ with (black columns) or without (dark-gray columns) IL-17A, for 2-24h as indicated. IκB-α and α-tubulin protein (a) were evaluated by western blot. Mean of optical density measurements of IκB-α (b) were corrected by α-tubulin. INS-1E cells (c) were co-transfected with a NF-κB luciferase reporter together with the pRL-CMV plasmid used as internal control. After 24h of recovery cells were left untreated or treated with IL-17A, combinations of IL-1β+IFN-γ with or without IL-17A, for 4h as indicated. Luciferase activity was assayed and corrected by the internal control. Results are mean ± SEM of 3-6 independent experiments. * p < 0.05 and *** p < 0.001 vs untreated cells of each time point; ANOVA followed by paired Student’s t-test with Bonferroni’s correction. (PDF 143 kb)
ESM Fig 3
STAT1 KD in INS-1E cells, HeLa cells, human islet cells and mouse islets KO for STAT1. INS-1E cells (a and b), HeLa cells (c and d) and dispersed human islets (e and f) were transfected with siCTRL (white columns) or with siSTAT1 (black columns). After 24 or 48h of recovery, cells were left untreated or treated with IL-17A, combinations of IL-1β+IFN-γ or TNF-α+IFN-γ with or without IL-17A, as indicated. Total (t)-STAT1 and α-tubulin protein (a and c) were evaluated by western blot. Mean of optical density measurements of t-STAT1 in INS-1E cells (b) and HeLa cells (d) were corrected by α-tubulin. t-STAT1 (e) and IRF-1 (f) mRNA expression was assayed by RT-PCR and normalized for the housekeeping gene β-actin. Mouse islets of w/t (C57Bl/6) (white columns) and Stat1 KO (black columns) mice (g) were left untreated or treated with IL-17A, combinations of IL-1β+IFN-γ or TNF-α+IFN-γ with or without IL-17A, as indicated. IRF-1 (g) mRNA expression was assayed by RT-PCR and normalized for three different housekeeping genes (β-actin, Rpl27 and Hprt). Results are mean ± SEM of 3 independent experiments for INS-1E cells or HeLa cells and 6 independent experiments for mouse and human islets. * p < 0.05, ** p < 0.01 and *** p < 0.001 vs untreated cells; † p < 0.05, †† p < 0.01 and ††† p < 0.001 and ‡‡‡ p < 0.001 as indicated; ANOVA followed by paired Student’s t-test with Bonferroni’s correction. (PDF 159 kb)
ESM Fig 4
STAT-1 KD affects cytokine-induced CXCL8 and IRF-7 mRNA expression in human islets. Human dispersed islets were transfected with siCTRL (white columns) or siSTAT1 (black columns). After 48h of recovery cells were left untreated or treated with IL-17A, combinations of IL-1β+IFN-γ or TNF-α+IFN-γ with or without IL-17A, for 48h as indicated. CXCL8 (a) and IRF-7 (b) mRNA expression was assayed by RT-PCR and normalized for the housekeeping gene β-actin. Results are mean ± SEM of 5-6 independent experiments. ** p < 0.01 and *** p < 0.001 vs untreated cells; † p < 0.05, ††† p < 0.001 and ‡‡‡ p < 0.001 as indicated; ANOVA followed by paired Student’s t-test with Bonferroni’s correction. (PDF 51 kb)
ESM Table 1
(PDF 119 kb)
ESM Table 2
(PDF 190 kb)
ESM Table 3
(PDF 120 kb)
ESM Table 4
(PDF 238 kb)
Rights and permissions
About this article
Cite this article
Grieco, F.A., Moore, F., Vigneron, F. et al. IL-17A increases the expression of proinflammatory chemokines in human pancreatic islets. Diabetologia 57, 502–511 (2014). https://doi.org/10.1007/s00125-013-3135-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-3135-2