Abstract
Aims/hypotheses
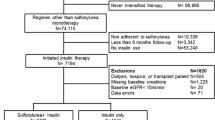
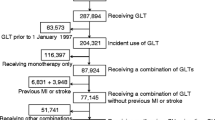
Despite oral hypoglycaemic medications being the most commonly used pharmacological treatments for type 2 diabetes, research is limited on their comparative safety, particularly their effects on overall mortality. We compared mortality risk with monotherapy initiation of four oral hypoglycaemic medications in a nationwide cohort of US veterans with type 2 diabetes.
Methods
We identified new users of oral hypoglycaemic medication monotherapy between 2004 and 2009 who received care for at least 1 year from the Veterans Health Administration. Patients were followed until initial monotherapy discontinuation, addition of another diabetes pharmacotherapy, death or end of follow-up. Mortality HRs were estimated using Cox regression adjusted for potential confounding factors.
Results
Among new users of metformin, sulfonylureas and rosiglitazone (185,360 men, 7,812 women), 4,256 (2.2%) died during follow-up. Average duration of medication use ranged from 1.4 to 1.7 years. Significantly higher mortality risk was seen for glibenclamide (known as glyburide in the USA and Canada) (HR 1.38, 95% CI 1.27, 1.50) or glipizide (HR 1.55, 95% CI 1.43, 1.67) compared with metformin monotherapy, and for glipizide compared with rosiglitazone (HR 1.27, 95% CI 1.01, 1.59) or glibenclamide monotherapy (HR 1.12, 95% CI 1.02, 1.23). A significant sex–rosiglitazone interaction was seen (p = 0.034) compared with metformin monotherapy, with women having a higher HR (HR 4.36, 95% CI 1.34, 14.20) than men (HR 1.19, 95% CI 0.95, 1.49).
Conclusions/interpretations
Significantly higher mortality was associated with glibenclamide, glipizide and rosiglitazone use compared with metformin, and with glipizide use compared with rosiglitazone or glibenclamide. The potential for residual confounding by indication should be considered in interpreting these results.

Similar content being viewed by others
Abbreviations
- ERIC:
-
Epidemiologic Research and Information Center
- VHA:
-
Veterans Health Administration
- VISN:
-
Veterans Integrated Service Network
References
CDC (2012) Age-adjusted percentage of adults with diabetes using diabetes medication, by type of medication, United States, 1997–2010. Available from www.cdc.gov/diabetes/statistics/meduse/fig2.htm. Accessed 20 July 2012
Qaseem A, Humphrey LL, Sweet DE, Starkey M, Shekelle P (2012) Oral pharmacologic treatment of type 2 diabetes mellitus: a clinical practice guideline from the American College of Physicians. Ann Intern Med 156:218–231
UK Prospective Diabetes Study (UKPDS) Group (1998) Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352:837–853
Chien HH, Chang CT, Chu NF et al (2007) Effect of glyburide-metformin combination tablet in patients with type 2 diabetes. J Chin Med Assoc 70:473–480
DeFronzo RA, Goodman AM (1995) Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 333:541–549
Garber AJ, Donovan DS Jr, Dandona P, Bruce S, Park JS (2003) Efficacy of glyburide/metformin tablets compared with initial monotherapy in type 2 diabetes. J Clin Endocrinol Metab 88:3598–3604
Goldstein BJ, Pans M, Rubin CJ (2003) Multicenter, randomized, double-masked, parallel-group assessment of simultaneous glipizide/metformin as second-line pharmacologic treatment for patients with type 2 diabetes mellitus that is inadequately controlled by a sulfonylurea. Clin Ther 25:890–903
Kahn SE, Haffner SM, Heise MA et al (2006) Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med 355:2427–2443
Meinert CL, Knatterud GL, Prout TE, Klimt CR (1970) A study of the effects of hypoglycemic agents on vascular complications in patients with adult-onset diabetes. II. Mortality results. Diabetes 19(Suppl):789–830
Nissen SE, Wolski K (2007) Effect of rosiglitazone on the risk of myocardial infarction and death from cardiovascular causes. N Engl J Med 356:2457–2471
Kaul S, Diamond GA (2011) Is there clear and convincing evidence of cardiovascular risk with rosiglitazone? Clin Pharmacol Ther 89:773–776
Winterstein AG (2011) Rosiglitazone and the risk of adverse cardiovascular outcomes. Clin Pharmacol Ther 89:776–778
Loke YK, Kwok CS, Singh S (2011) Comparative cardiovascular effects of thiazolidinediones: systematic review and meta-analysis of observational studies. BMJ 342:d1309
Nissen SE, Wolski K (2010) Rosiglitazone revisited: an updated meta-analysis of risk for myocardial infarction and cardiovascular mortality. Arch Intern Med 170:1191–1201
Singh S, Loke YK, Furberg CD (2007) Long-term risk of cardiovascular events with rosiglitazone: a meta-analysis. JAMA 298:1189–1195
Azoulay L, Schneider-Lindner V, Dell'aniello S, Schiffrin A, Suissa S (2010) Combination therapy with sulfonylureas and metformin and the prevention of death in type 2 diabetes: a nested case-control study. Pharmacoepidemiol Drug Saf 19:335–342
Eurich DT, Majumdar SR, McAlister FA, Tsuyuki RT, Johnson JA (2005) Improved clinical outcomes associated with metformin in patients with diabetes and heart failure. Diabetes Care 28:2345–2351
Evans JM, Ogston SA, Emslie-Smith A, Morris AD (2006) Risk of mortality and adverse cardiovascular outcomes in type 2 diabetes: a comparison of patients treated with sulfonylureas and metformin. Diabetologia 49:930–936
Gulliford M, Latinovic R (2004) Mortality in type 2 diabetic subjects prescribed metformin and sulphonylurea drugs in combination: cohort study. Diabetes Metab Res Rev 20:239–245
Johnson JA, Majumdar SR, Simpson SH, Toth EL (2002) Decreased mortality associated with the use of metformin compared with sulfonylurea monotherapy in type 2 diabetes. Diabetes Care 25:2244–2248
Schramm TK, Gislason GH, Vaag A et al (2011) Mortality and cardiovascular risk associated with different insulin secretagogues compared with metformin in type 2 diabetes, with or without a previous myocardial infarction: a nationwide study. Eur Heart J 32:1900–1908
Simpson SH, Majumdar SR, Tsuyuki RT, Eurich DT, Johnson JA (2006) Dose-response relation between sulfonylurea drugs and mortality in type 2 diabetes mellitus: a population-based cohort study. CMAJ 174:169–174
Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158:915–920
(2009) Selected Veterans Health Administration Characteristics: FY2002 to FY2011. Available from www.va.gov/vetdata/Utilization.asp. Accessed 20 Aug 2012
Cowper DC, Kubal JD, Maynard C, Hynes DM (2002) A primer and comparative review of major US mortality databases. Ann Epidemiol 12:462–468
Schafer JL (1999) Multiple imputation: a primer. Stat Methods Med Res 8:3–15
Gangji AS, Cukierman T, Gerstein HC, Goldsmith CH, Clase CM (2007) A systematic review and meta-analysis of hypoglycemia and cardiovascular events: a comparison of glyburide with other secretagogues and with insulin. Diabetes Care 30:389–394
Roumie CL, Hung AM, Greevy RA et al (2012) Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus, a cohort study. Ann Intern Med 157:601–610
Tzoulaki I, Molokhia M, Curcin V et al (2009) Risk of cardiovascular disease and all cause mortality among patients with type 2 diabetes prescribed oral antidiabetes drugs: retrospective cohort study using UK general practice research database. BMJ 339:b4731
Kahler KH, Rajan M, Rhoads GG et al (2007) Impact of oral antihyperglycemic therapy on all-cause mortality among patients with diabetes in the Veterans Health Administration. Diabetes Care 30:1689–1693
Home PD, Pocock SJ, Beck-Nielsen H et al (2009) Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet 373:2125–2135
Graham DJ, Ouellet-Hellstrom R, MaCurdy TE et al (2010) Risk of acute myocardial infarction, stroke, heart failure, and death in elderly Medicare patients treated with rosiglitazone or pioglitazone. JAMA 304:411–418
Juurlink DN, Gomes T, Lipscombe LL, Austin PC, Hux JE, Mamdani MM (2009) Adverse cardiovascular events during treatment with pioglitazone and rosiglitazone: population based cohort study. BMJ 339:b2942
Lipscombe LL, Gomes T, Levesque LE, Hux JE, Juurlink DN, Alter DA (2007) Thiazolidinediones and cardiovascular outcomes in older patients with diabetes. JAMA 298:2634–2643
Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL (2012) Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care 35:299–304
Stevens RJ, Ali R, Bankhead CR et al (2012) Cancer outcomes and all-cause mortality in adults allocated to metformin: systematic review and collaborative meta-analysis of randomised clinical trials. Diabetologia 55:2593–2603
Walker AM (1996) Confounding by indication. Epidemiology 7:335–336
Cummings P (2008) Propensity scores. Arch Pediatr Adolesc Med 162:734–737
Shah BR, Laupacis A, Hux JE, Austin PC (2005) Propensity score methods gave similar results to traditional regression modeling in observational studies: a systematic review. J Clin Epidemiol 58:550–559
Sturmer T, Joshi M, Glynn RJ, Avorn J, Rothman KJ, Schneeweiss S (2006) A review of the application of propensity score methods yielded increasing use, advantages in specific settings, but not substantially different estimates compared with conventional multivariable methods. J Clin Epidemiol 59:437–447
Brown DW (2010) Smoking prevalence among US veterans. J Gen Intern Med 25:147–149
Psaty BM, Weiss NS, Furberg CD et al (1999) Surrogate end points, health outcomes, and the drug-approval process for the treatment of risk factors for cardiovascular disease. JAMA 282:786–790
Acknowledgements
The Seattle Epidemiologic Research and Information Center (ERIC) is part of the Cooperative Studies Program of the Department of Veterans Affairs Office of Research and Development, Washington, DC, USA. The content of this article does not necessarily represent the views of the Department of Veterans Affairs or the US Government. We are grateful to S. E. Kahn, VA Puget Sound in Seattle, for his careful reading of and suggestions for this manuscript.
Funding
This work was supported by the ERIC of VA Puget Sound in Seattle, and by facilities and services provided by the Diabetes Research Center (DK-17047) at the University of Washington. VA Puget Sound Health Care System provided support for the involvement of E. J. Boyko, N. L. Smith and S. Wheeler in this research. J. S. Floyd was supported by National Heart, Lung and Blood Institute training grant T32 HL007902.
Duality of interest
The authors declare that there is no duality of interest associated with this manuscript.
Contribution statement
SW and EJB made substantial contributions to the conception and design of the study, drafted the article and provided approval for the final version. KM and KR acquired the data, revised the article critically for important intellectual content and provided approval for the final version. CWF, JSF and NLS analysed and interpreted the data, revised the article critically for important intellectual content and provided approval for the final version. EJB and SW had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM Table 1
PDF 26 kb
Rights and permissions
About this article
Cite this article
Wheeler, S., Moore, K., Forsberg, C.W. et al. Mortality among veterans with type 2 diabetes initiating metformin, sulfonylurea or rosiglitazone monotherapy. Diabetologia 56, 1934–1943 (2013). https://doi.org/10.1007/s00125-013-2958-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-013-2958-1