Abstract
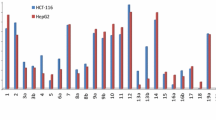
9-Amino acridine derivatives (1a–e) on condensation with dihydrofuran-2,5-dione, hexahydroisobenzofuran-1,3-dione and isochroman-1,3-dione under microwave irradiation gave corresponding condensation products 2a–e, 3a–e and 4a–e, respectively, in good yields. All these compounds were screened in vitro for anticancer activity against five human cancer cell lines, i.e., breast (T47D), lung (NCl H-522), colon (HCT-15), ovary (PA-1) and liver (Hep G2). Compounds 1-(3-methoxyacridine-9-yl) pyrrolidine-2,5-dione (2d) (ovary PA-1), 2-(2-methylacridine-9-yl)hexahydro-1H-isoindole-1,3(2H)-dione (3b) (lung NCl H-522), 2-(4-methylacridine-9-yl)hexahydro-1H-isoindole-1,3(2H)-dione (3c) (ovary PA-1), 2-(4-methoxyacridine-9-yl)hexahydro-1H-isoindole-1,3(2H)-dione (3e) (ovary PA-1) and 2-(2-methylacridine-9-yl)isoquinoline-1,3(2H,4H)-dione (4b) (ovary PA-1) exhibited IC50 values 7.1, 8.0, 5.4, 10.0 and 6.56 µM, respectively, and hence possess good anticancer activity.




Similar content being viewed by others
References
Abdel-Aziz AA-M, El-Tahir KEH, Asiri YA (2011) Synthesis, anti-inflammatory activity and COX-1/COX-2 inhibition of novel substituted cyclic imides. Part 1: molecular docking study. Eur J Med Chem 46:1648–1655
Albert A, Gledhill W (1945) Improved syntheses of aminoacridines. IV. Substituted 9-aminoacridines. J Soc Chem Ind L 64:169–172
Albert A, Ritchie B (1960) 9-aminoacridine. Org Synth Coll 3:53–56
Allen CFH, Mckee GHW (1959) Acridone. Org Synth Coll 2:15–17
Al-Suwaidan IA, Alanazi AM, El-Azab AS, Al-Obaid AM, El-Tahir KEH, Maarouf AR, El-Enin MAA, Abdel-Aziz AA-M (2013) Molecular design, synthesis and biological evaluation of cyclic imides bearing benzenesulfonamide fragment as potential COX-2 inhibitors. Part 2. Bioorg Med Chem Lett 23:2601–2605
Arya S, Kumar N, Roy P, Sondhi SM (2013a) Synthesis of amidine and bis amidine derivatives and their evaluation for anti-inflammatory and anticancer activity. Eur J Med Chem 59:7–14
Arya S, Kumar S, Rani R, Kumar N, Roy P, Sondhi SM (2013b) Synthesis, anti-inflammatory, and cytotoxicity evaluation of 9,10-dihydroanthracene-9,10-α, β-succinimide and bis-succinimide derivatives. Med Chem Res 22:4278–4285
Bacherikov VA, Chou T-C, Dong H-J, Zhang X, Chen C-H, Lin Y-W, Tsai T-J, Lee R-Z, Liu LF, Su T-L (2005) Potent antitumor 9-anilinoacridines bearing an alkylating N-mustard residue on the anilino ring: synthesis and biological activity. Bioorg Med Chem 13:3993–4006
Byun A, Park JY, Moon KH, Park MS (2008) Synthesis and hypnotic activities of N-Cbz-α-aminoglutarimidooxy carboxylate derivatives. Arch Pharm Res 31:834–837
Daghigh LR, Pordel M, Davoodnia A (2014) Synthesis of new fluorescent pyrazolo[4,3-a]acridine derivatives having strong antibacterial activities. J Chem Res 38(4):202–207
de Oliveira KN, Chiaradia LD, Martins PGA, Mascarello A, Cordeiro MNS, Guido RVC, Andricopulo AD, Yunes RA, Nunes RJ, Vernal J, Terenzi H (2011) Sulfonyl-hydrazones of cyclic imides derivatives as potent inhibitors of the Mycobacterium tuberculosis protein tyrosine phosphatase B (PtpB). Med Chem Commun 2:500–504
de Oliveira KN, Souza MM, Sathler PC, Magalhaes UO, Rodrigues CR, Castro HC, Palm PR, Sarda M, Perotto PE, Cezar S, de Brito MA, Ferreira ASSR, Cabral LM, Machado C, Nunes RJ (2012) Sulphonamide and sulphonyl-hydrazone cyclic imide derivatives: antinociceptive activity, molecular modeling and In Silico ADMET screening. Arch Pharm Res 35:1713–1722
Desbois N, Gardette M, Papon J, Labarre P, Maisonial A, Auzeloux P, Lartigue C, Bouchon B, Debiton E, Blache Y, Chavignon O, Teulade J-C, Maublant J, Madelmont J-C, Moins N, Chezal J-M (2008) Design, synthesis and preliminary biological evaluation of acridine compounds as potential agents for a combined targeted chemo-radionuclide therapy approach to melanoma. Bioorg Med Chem 16:7671–7690
El-Azab AS, Alanazi AM, Abdel-Aziz NI, Al-Suwaidan IA, El-Sayed MAA, El-Sherbeny MA, Abdel-Aziz AA-M (2013) Synthesis, molecular modeling study, preliminary antibacterial, and antitumor evaluation of N-substituted naphthalimides and their structural analogues. Med Chem Res 22:2360–2375
El-Deiry WS (2008) Acridine compound activation of p53 and use for the treatment of cancer. PCT Int Appl WO 2008010984 A2 20080124. Chem Abstr 148:160110
El-Zahabi MA, Gad LM, Bamanie FH, Al-Marzooki Z (2012) Synthesis of new cyclic imides derivatives with potential hypolipidemic activity. Med Chem Res 21:75–84
Gardette M, Viallard C, Paillas S, Guerquin-Kern J-L, Papon J, Moins N, Labarre P, Desbois N, Wong-Wah-Chung P, Palle S, Wu T-D, Pouget J-P, Miot-Noirault E, Chezal J-M, Degoul F (2014) Evaluation of two 125I-radiolabeled acridine derivatives for Auger-electron radionuclide therapy of melanoma. Invest New Drugs 32:587–597
Goodell JR, Madhok AA, Hiasa H, Ferguson DM (2006) Synthesis and evaluation of acridine- and acridone-based anti-herpes agents with topoisomerase activity. Bioorg Med Chem 14:5467–5480
Guetzoyan L, Yu X-M, Ramiandrasoa F, Pethe S, Rogier C, Pradines B, Cresteil T, Perree-Fauvet M, Mahy J-P (2009) Antimalarial acridines: Synthesis, in vitro activity against P. falciparum and interaction with hematin. Bioorg Med Chem 17:8032–8039
Hummersone MG, Cousin D, Frigerio M (2012) Acridine derivatives as telomerase inhibitors and their preparation and use for the treatment of proliferative disorders. PCT Int Appl WO 2012042265 A1 20120405. Chem Abstr 156:477677
Ip NY, Ip FCF, Hu Y, Han Y, Chung SK (2008) Preparation of acridine derivatives as cholinesterase inhibitors. PCT Int Appl WO 2008091901 A1 20080731. Chem Abstr 149:224114
Janovec L, Kozurkova M, Sabolova D, Ungvarsky J, Paulikova H, Plsikova J, Vantova Z, Imrich J (2011) Cytotoxic 3,6-bis((imidazolidinone)imino)acridines: synthesis, DNA binding and molecular modeling. Bioorg Med Chem 19:1790–1801
Kumar S, Kumar N, Roy P, Sondhi SM (2013a) Efficient synthesis of piperazine-2,6-dione and 4-(1H-indole-2-carbonyl)piperazine-2,6-dione derivatives and their evaluation for anticancer activity. Med Chem Res 22:4600–4609
Kumar S, Kumar N, Roy P, Sondhi SM (2013b) Synthesis, anti-inflammatory, and antiproliferative activity evaluation of isoindole, pyrrolopyrazine, benzimidazoisoindole, and benzimidazopyrrolopyrazine derivatives. Mol Divers 17:753–766
Kumar S, Kumar N, Roy P, Sondhi SM (2014) Efficient synthesis of heterocyclic compounds derived from 2,6-dioxopiperazine derivatives and their evaluation for anti-inflammatory and anticancer activities. Med Chem Res 23:3953–3969
Kumari G, Singh RK (2014) Green synthesis, antibacterial activity, and SAR of some novel naphthalimides and allylidenes. Med Chem Res. doi:10.1007/s00044-014-1118-6
Lang X, Li L, Chen Y, Sun Q, Wu Q, Liu F, Tan C, Liu H, Gao C, Jiang Y (2013) Novel synthetic acridine derivatives as potent DNA-binding and apoptosis-inducing antitumor agents. Bioorg Med Chem 21:4170–4177
Li Q, Fang H, Wang X, Xu W (2010) Novel cyclic-imide peptidomimetics as aminopeptidase N inhibitors. Structure-based design, chemistry and activity evaluation. II. Eur J Med Chem 45:1618–1626
Machado KE, de Oliveira KN, Santos-Bubniak L, Licinio MA, Nunes RJ, Santos-Silva MC (2011) Evaluation of apoptotic effect of cyclic imide derivatives on murine B16F10 melanoma cells. Bioorg Med Chem 19:6285–6291
Mcevoy GK (ed) (1992) AHFS drug information 484–610
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Prabakaran K, Yamuna E, Prasad KJR (2011) Synthesis and antimicrobial activities of nitro substituted indolo[3,2-c]acridines. Indian J Chem Sect B 50B:906–909
Sedlacek O, Hruby M, Studenovsky M, Vetvicka D, Svoboda J, Kankova D, Kovar J, Ulbrich K (2012) Polymer conjugates of acridine-type anticancer drugs with pH-controlled activation. Bioorg Med Chem 20:4056–4063
Sharma G, Park JY, Park MS (2008a) Design and synthesis of 6-amino-1,4-oxazepane-3,5-dione derivatives as novel broad spectrum anticonvulsants. Bioorg Med Chem Lett 18:3188–3191
Sharma G, Park JY, Park MS (2008b) Synthesis and anticonvulsant evaluation of 6-amino-1,4-oxazepane-3,5-dione derivatives. Arch Pharm Res 31:838–842
Su T-L, Lin Y-W, Chou T-C, Zhang X, Bacherikov VA, Chen C-H, Liu LF, Tsai T-J (2006) Potent antitumor 9-anilinoacridines and acridines bearing an alkylating N-mustard residue on the acridine chromophore: synthesis and biological activity. J Med Chem 49:3710–3718
Szymanski P, Markowicz M, Bajda M, Malawska B, Mikicuik-Olasik E (2012) Synthesis, biological activity and molecular modeling of 4-fluoro-N-[ω-(1,2,3,4-tetrahydroacridin-9-ylamino)-alkyl]-benzamide derivatives as cholinesterase inhibitors. Arzneim Forsch 62(12):655–660
Teitelbaum AM, Gallardo JL, Bedi J, Giri R, Benoit AR, Olin MR, Morizio KM, Ohlfest JR, Remmel RP, Ferguson DM (2012) 9-Amino acridine pharmacokinetics, brain distribution, and in vitro/in vivo efficacy against malignant glioma. Cancer Chemother Pharmacol 69:1519–1527
Thi HTN, Lee C-Y, Teruya K, Ong W-Y, Doh-ura K, Go M-L (2008) Antiprion activity of functionalized 9-aminoacridines related to quinacrine. Bioorg Med Chem 16:6737–6746
Tomer V, Bhattacharjee G, Kamaluddin Rajakumar S, Srivastava K, Puri SK (2010) Synthesis of new chalcone derivatives containing acridinyl moiety with potential antimalarial activity. Eur J Med Chem 45:745–751
Tonelli M, Vettoretti G, Tasso B, Novelli F, Boido V, Sparatore F, Busonera B, Ouhtit A, Farci P, Blois S, Giliberti G, Colla PL (2011) Acridine derivatives as anti-BVDV agents. Antivir Res 91:133–141
Villa V, Tonelli M, Thellung S, Corsaro A, Tasso B, Novelli F, Canu C, Pino A, Chiovitti K, Paludi D, Russo C, Sparatore A, Aceto A, Boido V, Sparatore F, Florio T (2011) Efficacy of novel acridine derivatives in the inhibition of hPrP90-231 prion protein fragment toxicity. Neurotox Res 19:556–574
Wang S-S, Lee Y-J, Hsu S-C, Chang H-O, Yin W-K, Chang L-S, Chou S-Y (2007) Linker-modified triamine-linked acridine dimers: synthesis and cytotoxicity properties in vitro and in vivo. Bioorg Med Chem 15:735–748
Yesildag B, Ulus R, Basar E, Aslan M, Kaya M, Bulbul M (2014) Facile, highly efficient, and clean one-pot synthesis of acridine sulfonamide derivatives at room temperature and their inhibition of human carbonic anhydrase isoenzymes. Monatsh Chem 145:1027–1034
Acknowledgments
We are thankful to technical staff of the Chemistry Department, I. I. T. Roorkee, for spectroscopic studies and elemental analysis. Thanks are also due to Head I.I.C. for providing NMR facility. Mr. Anuj Kumar is thankful to MHRD, New Delhi, for financial assistance.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kumar, A., Kumar, N., Roy, P. et al. Synthesis of acridine cyclic imide hybrid molecules and their evaluation for anticancer activity. Med Chem Res 24, 3272–3282 (2015). https://doi.org/10.1007/s00044-015-1380-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-015-1380-2