Abstract
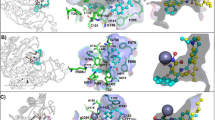
This work describes the effect of two series of isoindolines 1(a–g) and 2(a–g) on histone deacetylase 8 (HDAC8) activity by theoretical study (docking method) and experimental assays using a commercial kit, as well as the effect of isoindolines on HDAC8 expression on HeLa cells. The theoretical study show interactions of the isoindolines with two HDAC8 deep pockets; these binding sites were compared to those of HDAC8 inhibitor, Trichostatin A. It was demonstrated experimentally that tested compounds can act as HDAC8 inhibitors. The expression of HDAC8 by Western blot analysis, remain unchanged in HeLa cells treated with the isoindolines with the exception of compound 2b which seems to increase HDAC8 expression. In conclusion, isoindolines act as HDAC8 inhibitors because they do not decrease the protein expression.





Similar content being viewed by others
References
Barlaam B, Dantzman C (2002) Preparation of isoquinolines and isoindolines as selective estrogen receptor-β ligand. WO 0246164
Berger D, Citarella R, Dutia M, Greenberger L, Hallet W, Paul R, Powell D (1999) Novel multidrug resistance reversal agents. J Med Chem 42:2145–2161. doi:10.1021/jm9804477
Bhagwat SS, Gayo F, Leah M, Stein BM, Chao Qi, Gangloff AR, McKie JA, Rice KD (2002) Preparation of tetrahydroisoquinolines, tetrahydrobenzazepines, and isoindolines as selective modulators of ER-β estrogen receptors for the treatment of estrogen-related conditions. WO 0224653
Chimenti F, Vomero S (1975) Synthesis of N-substituted isoindolines. Farmaco Sci 30:884–890
Kukkola PJ, Bilci NA, Ikler T, Savage P, Shetty SS, DelGrande D, Jeng AY (2001) Isoindolines: a new series of potent and selective endothelin-A receptor antagonists. Bioorg Med Chem Lett 11:1737–1740. doi:10.1016/S0960-894x(01)00273-6
Kurys BE, Fink DM, Freed BS, Meriman GH (1998) N-(pyridinylamino)isoindolines for treating Alzheimer’s and depression. WO1998029407
Lee S, Shinji C, Ogura K, Shimizu M, Maeda S, Sato M, Yoshida M, Hashimoto Y, Miyachi H (2007) Design, synthesis, and evaluation of isoindolinone-hydroxamic acid derivatives as histone deacetylase (HDAC) inhibitors. Bioorg Med Chem Lett 17:4895–4900. doi:10.1016/j.bmcl.2007.06.038
Lin HY, Chen CS, Lin SP, Weng JR, Chen CS (2006) Targeting histone deacetylase in cancer therapy. Med Res Rev 26:397–413. doi:10.1002/med.20056
Maier T, Beckers T, Hesslinger C (2009) Preparation of tetrahydroisoquinoline/isoindoline hydroxamic acids as HDAC6 histone deacetylase inhibitors. WO 2009-EP52924
Mancilla Percino T, Correa Basurto J, Trujillo Ferrara J, Ramos Morales FR, Acosta Hernández ME, Cruz-Sánchez JS, Saavedra-Vélez M (2010) Molecular modeling study of isoindolines as l-type Ca2+ channel blockers by docking calculations. J Mol Model 16:1377–1382. doi:10.1007/s00894-010-0643-6
Mancilla T, Carrillo L, Zamudio L, Beltran HI, Farfan N (2001) Synthesis and characterization of new 2-substituted isoindoline derivatives of α-amino acids. Org Prep Proc Int 33:341–349. doi:10.1080/00304940109356598
Mancilla T, Correa-Basurto J, Alavés Carbajal KS, Sánchez Escalante ETJ, Trujillo Ferrara J (2007) Theoretical study of isoindolines to identify them as cyclooxigenase-1 and -2 inhibitors by docking simulation. J Mex Chem Soc 51:95–102 ISSN1870-249X
Mertens A, Zilch H, Koenig B, Schaefer W, Poll T, Kampe W, Seidel H, Leser U, Leinert H (1993) Selective non-nucleoside HIV-1 reverse transcriptase inhibitors. New 2,3-dihydrothiazolo(2,3-a)isoindol-5(9bH)-ones and related compounds with anti-HIV-1 activity. J Med Chem 36:2526–2535. doi:10.1021/jm00069a011
Muller GW, Stirling DI, Chen RS, Man H (1999a) Preparation of 2-(2,6-dioxo-3-fluoropiperidin-3-yl) isoindolines for reducing inflammatory cytokine levels. US 98-42274
Muller GW, Stirling DI, Chen RS, Man H (1999b) Preparation of 2-(2, 6-dioxo-3-fluoropiperidin-3-yl) isoindolines and their use to reduce tumor necrosis factor α levels. WO 98-US24453
Muller GW, Stirling DI, Man H (2008) Pharmaceutically active isoindoline derivatives. TW 2001-90128385
Robarge MJ, Chen RS, Muller GW, Man H (2001) Isoindoline derivates as TNF-inhibitors. EP2168958
Schrump DS (2009) Cytotoxicity mediated by histone deacetylase inhibitors in cancer cells: mechanisms and potential clinical implications. Clin Cancer Res 15:3947–3957. doi:10.1158/1078-0432.CCR-08-2787
Shinji C, Nakamura T, Maeda S, Yoshida M, Hashimoto Y, Miyachi H (2005) Design and synthesis of phthalimide-type histone deacetylase inhibitors. Bioorg Med Chem Lett 15:4427–4431. doi:10.1016/j.bmcl.2005.07.048
Trejo Muñoz CR, Mancilla Percino T, Mera Jiménez E, Correa-J, Trujillo Ferrara JG (2012) Partition coefficient determination of a series of isoindolines-2-substituted and its correlation with their antiproliferative activity on HeLa cells. Med Chem Res. http://link.springer.com/article/10.1007%2Fs00044-012-0399-x. Accessed 06 April 2013
Witt O, Hedwig ED, Milde T, Oehme I (2009) HDAC family: what are the cancer relevant targets? Cancer Lett 277:8–21. doi:10.1016/j.canlet.2008.08.016
MDL Information Systems Inc. (2002) ISIS/Draw, v.2.3 and 2.5. MDL Information Systems, Inc (now Symyx Technologies, Inc.), San Leandro
Buggy JJ, Sideris ML, Mak P, Lorimer DD, McIntosh B, Clark JM (2000) Cloning and characterization of a novel human histone deacetylase, HDAC8. Biochem J 350:199–205. doi:10.1074/jbc.M908988199
Estiu G, West N, Mazitschek R, Greenberg E, Bradner JE (2010) On the inhibition of histone deacetylase 8. Bioorg Med Chem 18:4103–4110. doi:10.1016/j.bmc.2010.03.080
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Zakrzewski VG, Montgomery JA, Stratmann RE, Burant JC, Dapprich S, Millam JM, Daniels AD, Kudin KN, Strain MC, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Peterson GA, Ayala PY, Cui Q, Morokuma K, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Cioslowski J, Ortiz JV, Baboul AG, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Andres JL, Gonzalez C, Head-Gordon M, Replogle ES, Pople JA (1998) Gaussian 98, Revision A.9, Gaussian, Inc, Pittsburgh, PA
Humprey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. doi:10.1016/0236-7855(96)00018-5
Jing H, Hongli L, Yan C (2006) Effects of trichostatin A on HDAC8 expression, proliferation and cell cycle of Molt-4 cells. J Huazhong Univ Sci Technol 26:531–533
Montero A, Beierle JM, Olsen CA, Ghadiri MR (2009) Design, synthesis, biological evaluation, and structural characterization of potent histone deacetylase inhibitors based on cyclic alpha/beta-tetrapeptide architectures. J Am Chem Soc 131:3033–3041. doi:10.1021/ja809508f
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662. doi:10.1002/(SICI)1096-987x(19981115
Oehme I, Deubzer HE, Wegener D, Pickert D, Linke JP, Hero B, Kopp-Schneider A, Westermann F, Ulrich SM, von Deimling A, Fischer M, Witt O (2009) Histone deacetylase 8 in neuroblastoma tumorigenesis. Clin Cancer Res 15:91–99. doi:10.1517/14728220903241658
Somoza JR, Skene RJ, Katz BA, Mol C, Ho JD, Jennings AJ, Luong C, Arvai A, Buggy JJ, Chi E, Tang J, Sang BC, Verner E, Wynands R, Leahy EM, Dougan DR, Snell G, Navre M, Knuth MW, Swanson RV, McRee DE, Tari WE (2004) Structural snapshots of human HDAC8 provide insights into the class I histone deacetylases. Structure 12:1325–1334. doi:10.1016/j.str.2004.04.012
Vannini A, Volpari C, Filocamo G, Casavola EC, Brunetti M, Renzoni D, Chakravarty P, de Francesco Paolini, Gallinari P, Steinkühler Ch, di Marco S (2004) Crystal structure of a eukaryotic zinc-dependent histone deacetylase, human HDAC8, complexed with a hydroxamic acid inhibitor. PNAS 101:15064–15069. doi:10.1073/pnas.0404603101
Acknowledgments
The authors are grateful to CONACYT for scholarship No. 215133 to CRTM, SIP-COFAA/IPN, CONACYT (132353), and ICyTDF for financial support, to Brenda Trejo for language help corrections.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Trejo Muñoz, C.R., Jiménez, E.M., Pinto-Almazán, R. et al. Effect of two series of isoindolines over HDAC8 activity and expression. Med Chem Res 23, 3227–3234 (2014). https://doi.org/10.1007/s00044-013-0903-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0903-y