Abstract
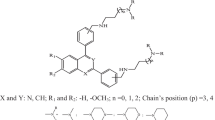
Quantitative structure activity relationship (QSAR) model for antimalarial activity was developed from a set of 51 substituted quinazolines that exhibited remarkable in vitro activity against sensitive and multidrug-resistant Plasmodium falciparum malaria. 2D-QSAR was done using partial least squares method coupled with stepwise variable selection; subsequently, 3D-QSAR was carried out using stepwise variable selection k-nearest neighbor molecular field analysis (kNNMF) approach. Leave-one-out cross-validated correlation coefficient (q 2) of 0.7601 and a predicted r 2 for the external test (pred_r 2) of 0.7108 were obtained with 3D QSAR. Experimental results revealed that the molecular weight, alignment-independent descriptors, electrostatic and steric field descriptors were significantly correlated with antimalarial activity of quinazoline derivatives. The results helped to understand the nature of substituents around quinazoline nucleus, thereby providing new guidelines for the design of novel antimalarials.








Similar content being viewed by others
References
Anson BD, Ma J, He JQ (2009) Identifying cardiotoxic compounds. Genet Eng Biotechnol 29:34–35
Aregawi M, Cibulskis R, Otten M, Williams R, Dye C (2008) World malaria report. World Health Organization
Halgren TA (1996) Merck molecular force field. III. Molecular geometries and vibrational frequencies for MMFF94. J Comput Chem 17:553–586
Meshnick SR (2002) Artemisinin: mechanisms of action, resistance and toxicity. Int J Parasitol 32:1655–1660
Paul SM, Mytelka DS, Dunwiddie CT, Persinger CC, Munos BH, Lindborg SR, Schacht AL (2010) How to improve R&D productivity: the pharmaceutical industry’s grand challenge. Nat Rev Drug Discov 9:203–214
Riegelhaupt PM, Frame IJ, Akabas MH (2010) Transmembrane segment 11 appears to line the purine permeation pathway of the Plasmodium falciparum equilibrative nucleoside transporter 1 (PfENT1). J Biol Chem 285:17001–17010
Verhaeghe P, Azas N, Gasquet M, Hutter S, Ducros C, Laget M, Rault S, Rathelot P, Vanelle P (2008) Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorg Med Chem Lett 18:396–401
Verhaeghe P, Azas N, Hutter S, Ducros C, Laget M, Dumetre A, Gasquet M, Reboul JP, Rault S, Rathelot P, Vanelle P (2009) Synthesis and in vitro antiplasmodial evaluation of 4-anilino-2-trichloromethylquinazolines. Bioorg Med Chem 17:4313–4322
Winstanley PA (2000) Chemotherapy for falciparum malaria: the armoury, the problems and the prospects. Parasitol Today 16:146–152
Xu M, Zhang A, Han S, Wang L (2002) Studies of 3d-quantitative structure–activity relationships on a set of nitroaromatic compounds: CoMFA, advanced CoMFA and CoMSIA. Chemosphere 48:707–715
Zhu S, Hudson TH, Kyle DE, Lin AJ (2002) Synthesis and in vitro studies of novel pyrimidinyl peptidomimetics as potential antimalarial therapeutic agents. J Med Chem 45:3491–3496
Acknowledgments
The author is grateful to Head, Department of Pharmacy, Barkatullah University, Bhopal, for providing the V-Life MDS software Version 3.5 and other necessary facilities for carrying out the Research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, M., Mishra, V.K., Senger, P. et al. Exploring QSAR studies on 4-substituted quinazoline derivatives as antimalarial compounds for the development of predictive models. Med Chem Res 23, 1397–1405 (2014). https://doi.org/10.1007/s00044-013-0744-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0744-8