Abstract
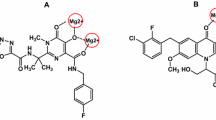
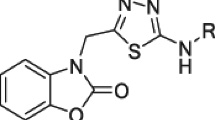
A new series of 4-oxo-4H-pyrido[1,2-a]pyrimidine derivatives containing 1,3,4-oxadiazole and 1,3,4-thiadiazole rings as a part of the metal chelation motif were synthesized and evaluated for their in vitro anti-HIV-1 activity. Most of the tested compounds displayed moderate inhibitory properties against HIV-1 virus (NL4-3) in Hela cell cultures. Compounds 11e and 11b exhibited the highest activity among the synthesized compounds with inhibition rate of 51 and 48 % at concentration of 100 μM, respectively. Molecular docking study using the later crystallographic data available for PFV integrase (IN) showed that the designed compounds bind into the active site of IN such that the keto oxygen atom at position of C-4 and nitrogen atom of thiadiazole or oxadiazole ring moiety chelate the Mg2+ ion. Our results also showed that all tested compounds presented no significant cytotoxicity at concentration of 100 μM. Therefore, these compounds can provide a very good basis for the development of new hits.




Similar content being viewed by others
References
Barbaro G, Scozzafava A, Mastrolorenzo A, Supuran CT (2005) Highly active antiretroviral therapy: current state of the art, new agents and their pharmacological interactions useful for improving therapeutic outcome. Curr Pharm Des 11:1805–1843
Charpentier C, Karmochkine M, Laureillard D, Tisserand P, Belec L, Weiss L, Si-Mohamed A, Piketty C (2008) Drug resistance profiles for the HIV integrase gene in patients failing raltegravir salvage therapy. HIV Med 9:765–770
Colicelli J, Goff SP (1988) Sequence and spacing requirements of a retrovirus integration site. J Mol Biol 199:47–59
Donghi M, Kinzel OD, Summa V (2009) 3-Hydroxy-4-oxo-4H-pyrido[1,2-a]pyrimidine-2-carboxylates-A new class of HIV-1 integrase inhibitors. Bioorg Med Chem Lett 19:1930–1934
Hare S, Gupta SS, Valkov E, Engelman A, Cherepanov P (2010) Retroviral intasome assembly and inhibition of DNA strand transfer. Nature 464:232–236
Herr J (2002) 5-Substituted-1H-terazoles as carboxylic acid isosteres: medicinal chemistry and synthetic methods. Bioorg Med Chem 10:3379–3393
Johns BA, Weatherhead JG, Allen SH, Thompson JB, Garvey EP, Foster SA, Jeffrey JL, Miller WH (2009) 1,3,4-Oxadiazole substituted naphthyridines as HIV-1 integrase inhibitors. Part 2: SAR of the C5 position. Bioorg Med Chem Lett 19:1807–1810
Kirschberg T, Parrish J (2007) Metal chelators as antiviral agents. Curr Opin Drug Discov Dev 10:460–472
Lin CC, Cheng HY, Yang CM, Lin TC (2002) Antioxidant and antiviral activities of the Euphorbia thymifolia Linn. J Biomed Sci 9:656–664
Miller MD, Farnet CM, Bushman FD (1997) Human immunodeficiency virus type 1 preintegration complexes: studies of organization and composition. J Virol 71:5382–5390
Min S, Song I, Borland J, Chen S, Lou Y, Fujiwara T, Piscitelli SC (2010) Pharmacokinetics and safety of S/GSK1349572, a nextgeneration HIV integrase inhibitor, in healthy volunteers. Antimicrob Agents Chemother 54(1):254–258
Morris GM, Goodsell DS, Halliday RS, Huey R, Hart WE, Belew RK, Olson AJ (1998) Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem 19:1639–1662
Moyle G, Gatell J, Perno CF, Ratanasuwan W, Schechter M, Tsoukas C (2008) Potential for new antiretrovirals to address unmet needs in the management of HIV-1 infection. AIDS Patient Care STDs 22:459–471
Pommier Y, Johnson AA, Marchand C (2005) Integrase inhibitors to treat HIV/AIDS. Nat Rev Drug Discov 4:236–248
Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, Currens MJ, Seniff D, Boyd MR (1998) Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res 48:4827–4833
Shimura K, Kodama E, Sakagami Y, Matsuzaki Y, Watanabe W, Yamataka K, Watanabe Y, Ohata Y, Doi S, Sato M, Kano M, Ikeda S, Matsuoka M (2008) Broad antiretroviral activity and resistance profile of the novel human immunodeficiency virus integrase inhibitor elvitegravir (JTK-303/GS-9137). J Virol 82(2):764–774
Zabihollahi R, Sadat SM, Vahabpour R, Aghasadeghi MR, Memarnejadian A, Ghazanfari T, Salehi M, Rezaei A, Azadmanesh K (2011) Development of single-cycle replicable human immunodeficiency virus 1 mutants. Acta Virol 55(1):15–22
Zhuang L, Wai JS, Embrey MW, Fisher TE, Egbertson MS, Payne LS, Guare JP, Vacca JP, Hazuda DJ, Felock PJ, Wolfe AL, Stillmock KA, Witmer MV, Moyer G, Schleif WA, Gabryelski LJ, Leonard YM, Lynch JJ, Michelson SR, Young SD (2003) Design and synthesis of 8-hydroxy-[1,6]naphthyridines as novel inhibitors of HIV-1 integrase in vitro and in infected cells. J Med Chem 46:453–456
Acknowledgments
This study was financially supported by Research Deputy of Shahid Beheshti University of Medical sciences as part of Ph.D. thesis of Z. Hajimahdi.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hajimahdi, Z., Zarghi, A., Zabihollahi, R. et al. Synthesis, biological evaluation, and molecular modeling studies of new 1,3,4-oxadiazole- and 1,3,4-thiadiazole-substituted 4-oxo-4H-pyrido[1,2-a]pyrimidines as anti-HIV-1 agents. Med Chem Res 22, 2467–2475 (2013). https://doi.org/10.1007/s00044-012-0241-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0241-5