Abstract
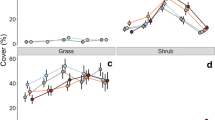
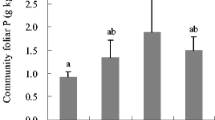
In floodplain primary succession, vegetation colonizes nitrogen-poor alluvial deposits and fertility improves as soil nitrogen accumulates over time. It is generally assumed that vegetation assimilates the vast majority of its nitrogen from the soil; however, recent studies have suggested that the hyporheic zone also may be an important nitrogen source. We investigated the potential relative importance of hyporheic nitrogen by comparing fertility indices, specifically total (TN), dissolved inorganic (DIN), potentially mineralizable (PMN) and ion exchange resin nitrogen (IERN) in both soils and the hyporheic zone at early, mid and late succession stands on an expansive river flood plain. We also constructed mesocosms to assess growth of cottonwood cuttings with access to soil and/or hyporheic water. We found TN and PMN increased from early to mid succession in both the soil (to 10 cm) and hyporheic zone (in a 10 cm layer). While TN, DIN and PMN were an order of magnitude higher in the soil than in the hyporheic zone, IERN was higher in the hyporheic zone, indicating that subsurface flow through the flood plain may be important in delivering nitrogen to the root zone. However, even when flux was added to the hyporheic PMN pool, nitrogen availability in the hyporheic zone (in a 10 cm layer) was vastly lower than soil PMN (to 10 cm). Further, the instantaneous standing stock of DIN in the surface soil alone was about equal to the sum of the DIN pool, the mean subsurface flux and the PMN pool in a 10 cm layer of hyporheic zone. In the mesocosm experiment, cottonwood cuttings with access to both soil and hyporheic water grew fastest; however, they also had the lowest foliar nitrogen concentrations, indicating that this was not due to greater nitrogen availability. In the field, nitrogen content of cottonwood foliage increased along with soil (but potentially hyporheic as well) nitrogen accumulation during succession, suggesting the vegetation responded to increasing nitrogen fertility. We conclude that at least on a per unit-volume basis, the hyporheic zone probably provides little nitrogen relative to the surface soil, except on new alluvial bars that characteristically are nitrogen poor. Therefore, the hyporheic zone is probably a much smaller nitrogen source for mature forests relative to the surface soil unless the vegetation exploits a much larger volume of the hyporheic zone than surface soil.



Similar content being viewed by others
References
Adair EC, Binkley D (2002) Co-limitation of first-year Fremont cottonwood seedlings by nitrogen and water. Wetlands 22:425–429
Adair EC, Binkley D, Anderson DC (2004) Patterns of nitrogen accumulation and cycling in riparian floodplain ecosystems along the Green and Yampa rivers. Oecologia 139:108–116
APHA (American Public Health Association) (2007) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, D.C. http://www.standardmethods.org/
Binkley D (1984a) Ion exchange resin bags: factors affecting estimates of nitrogen availability. Soil Sci Soc Am J 48:1181–1184
Binkley D (1984b) Ion exchange resin bags for assessing soil N availability: the importance of ion concentration, water regime, and microbial competition. Soil Sci Soc Am J 48:1181–1184
Binkley D, Hart SC (1989) The components of nitrogen availability assessments in forest soils. Adv Soil Sci 10:58–111
Binkley D, Cromack K Jr, Baker DD (1994) Nitrogen fixation by red alder: biology, rates and controls. In: Hibbs DE, DeBell DS, Tarrant RF (eds) The biology and management of red alder. Oregon State University Press, Corvallis, pp 57–72
Boggs K, Weaver T (1994) Changes in vegetation and nutrient pools during riparian succession. Wetlands 14:98–109
Boone RD, Grigal DF, Sollins P, Ahrens RJ, Armstrong DE (1999) Soil sampling, preparation, archiving, and quality control. In: Robertson PG, Coleman DC, Bledsoe CS, Sollins P (eds) Standard soil methods for long-term ecological research. Oxford University Press, New York, pp 3–28
Boulton AJ, Daltry T, Kasahara T, Mutz M, Stanford JA (2010) Ecology and management of the hyporheic zone: stream–groundwater interactions of running waters and their floodplains. J North Am Benthol Soc 29(1):26–40
Braatne JH, Rood SB, Heilman PR (1996) Life history ecology and conservation of riparian cottonwoods in North America. In: Stettler R, Bradshaw T, Heilman P, Hinckley T (eds) Biology of Populus and its implications for management and conservation. NRC Research Press, National Research Council of Canada, Ottawa, pp 57–85
Bundy LG, Meisinger JJ (1994) Nitrogen availability indices. In: Weaver RW, Angle S, Bottomly P, Bezdicek D, Smith S, Tabatabai A, Wollum A (eds) Methods of soil analysis. Part 2., Soil Science Society of America Book Series 5Soil Science Society of America, Madison, pp 951–984
Chapin FS III, Matson PA, Mooney HA (2002) Terrestrial plant nutrient use, chapter 8. In: Principles of terrestrial ecosystem ecology. Springer, New York, pp 176–195
Claret C, Marmonier P, Boissier JM, Fontvieille D, Blanc P (1997) Nutrient transfer between parafluvial interstitial water and river water: influence of gravel bar heterogeneity. Freshw Biol 37:657–670
Cliverd H, Jones JB Jr, Kielland K (2008) Nitrogen retention in the hyporheic zone of a glacial river in interior Alaska. Biogeochemistry 88:31–46
Cottam G, Curtis JT (1956) The use of distance measures in phytosociological sampling. Ecology 37:451–460
Diehl JC (2004) Hydrogeological characteristics and groundwater/river exchange in a gravel-dominated floodplain, Middle Fork of the Flathead River, northwestern Montana. Masters thesis, The University of Montana, Missoula, MT, USA
Doty SL, Dosher MR, Singleton GL, Moore AL, van Aken B, Stettler RF, Strand SE, Gordon MP (2005) Identification of endophytic Rhizobium in stems of Populus. Symbiosis 39:27–35
Ellis BK (2006) Alternate states in a large oligotrophic lake: a retrospective analysis of nutrient loading and food web change. Dissertation, The University of Montana, Missoula, MT, USA
Fastie CL (1995) Causes and ecosystem consequences of multiple pathways of primary succession at Glacier Bay, Alaska. Ecology 76:1899–1916
Fetter CW (2001) Applied hydrogeology, 4th edn. Prentice Hall, Englewood Cliffs
Graphpad Prism 5 (2007) Graphpad Software, San Diego, CA, USA
Gurnell A, Petts G (2006) Trees as riparian engineers: the Tagliamento River, Italy. Earth Surf Proc Land 31:1558–1574
Harner MJ, Stanford JA (2003) Differences in cottonwood growth between a losing and gaining reach of an alluvial floodplain. Ecology 84:1453–1458
Harner MJ, Ramsey PW, Rillig MC (2004) Protein accumulation and distribution in floodplain soils and river foam. Ecol Lett 7:829–836
Harner MJ, Mummey DL, Stanford JA, Rillig MC (2010) Arbuscular mycorrhizal fungi enhance spotted knapweed growth across a riparian chronosequence. Biol Invasions 12:1481–1490
Hart SC, Binkley D (1985) Correlations among indices of forest soil nutrient availability in fertilized and unfertilized loblolly pine plantations. Plant Soil 85:11–21
Hefting M, Clément JC, Dowrick D, Cosandey AC, Bernal S, Cimpian C, Tatur A, Burt TP, Pinay G (2004) Water table elevation controls soil nitrogen cycling in riparian wetlands along a European climatic gradient. Biogeochemistry 67:113–134
Hill AR (1996) Nitrate removal in stream riparian zones. J Environ Qual 25:743–755
Johnson DW, Verburg PSJ, Arnone JA (2005) Soil extraction, ion exchange resin, and ion exchange membrane measures of soil mineral nitrogen during incubation of a tallgrass prairie soil. Soil Sci Soc Am J 69:260–265
Kielland KJ, Olson K, Ruess RW, Boone RD (2006) Contribution of winter processes to soil nitrogen flux in taiga forest ecosystems. Biogeochemistry 81:349–360
Latterell JJ, Bechtold JS, O’Keefe TC, van Pelt R, Naiman RJ (2006) Dynamic patch mosaics and channel movement in an unconfined river valley of the Olympic Mountains. Freshw Biol 51:523–544
Lisuzzo NJ, Kielland K, Jones JB (2008) Hydrologic controls on nitrogen availability in a high-latitude, semi-arid floodplain. Ecoscience 15:366–376
Lorang MS, Hauer FR (2006) Fluvial geomorphic processes, chapter 7. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology, 2nd edn. Academic Press/Elsevier, San Diego, pp 145–168
Lowrance R, Todd R, Frail J Jr, Hendrickson O Jr, Leonard R, Asmussen L (1984) Riparian forests as nutrient filters in agricultural watersheds. Bioscience 34:374–377
Luken JO, Fonda RW (1983) Nitrogen accumulation in a chronosequence of red alder communities along the Hoh River, Olympic National Park, Washington. Can J For Res 13:1228–1237
Mouw JEB, Alaback PB (2003) Putting floodplain hyperdiversity in a regional context: an assessment of terrestrial-floodplain connectivity in a montane environment. J Biogeogr 30:87–103
Mouw JEB, Stanford JA, Alaback PB (2009) Influences of flooding and hyporheic exchange on floodplain plant richness and productivity. River Res Appl 25:929–945
Nadelhoffer KJ, Giblin AE, Shaver GR, Laundre JA (1991) Effects of temperature and substrate quality on element mineralization in six arctic soils. Ecology 72:242–253
Nakamura F, Shin N, Inahara S (2007) Shifting mosaic in maintaining diversity of floodplain tree species in the northern temperate zone of Japan. For Ecol Manage 241:28–38
NOAA (National Climate Data Center) (2007) COOP Data/Record of River and Climatological Observations West Glacier Station, MT, USA. Retrieved on 14 May 2010. http://www.ncdc.noaa.gov/oa/ncdc.html
Peterjohn WT, Correll DL (1984) Nutrient dynamics in an agricultural watershed: observations on the role of a riparian forest. Ecology 65:1466–1475
Pinay G, Ruffinoni C, Wondzell S, Gazelle F (1998) Change in groundwater nitrate concentration in a large river floodplain: denitrification, uptake or mixing? J North Am Benthol Soc 17:179–189
Piotrowski, JS (2007) Succession of arbuscular mycorrhizal fungi: causes, consequences, and considerations. Dissertation, The University of Montana, Missoula, MT, USA
Piotrowski JS, Lekberg Y, Harner MJ, Ramsey PW, Rillig MC (2008) Dynamics of mycorrhizae during development of riparian forests along an unregulated river. Ecography 31:245–253
Reid BL (2007) Energy flow in a floodplain aquifer ecosystem. Dissertation, The University of Montana, Missoula, MT, USA
Rood SB, Braatne JH, Hughes FMR (2003) Ecophysiology of riparian cottonwoods: stream flow dependency water relations and restoration. Tree Physiol 23:1113–1124
Sasaki A, Fujiyoshi M, Shidara S, Nakatsubo T (2001) Effects of nutrients and arbuscular mycorrhizal colonization on the growth of Salix gracilistyla seedlings in a nutrient-poor fluvial bar. Ecol Res 16:165–172
Schade JD, Marti E, Welter JR, Fisher SG, Grimm NB (2002) Sources of nitrogen to the riparian zone of a desert stream: implications for riparian vegetation and nitrogen retention. Ecosystems 5:68–79
Schade JD, Welter JR, Marti E, Grimm NB (2005) Hydrologic exchange and N uptake by riparian vegetation in an arid-land stream. J North Am Benthol Soc 24:19–28
Spalding RF, Exner ME (1993) Occurrence of nitrate in groundwater—a review. J Environ Qual 22:392–402
Stanford G (1982) Assessment of soil nitrogen availability. In: Stevenson FJ (ed) Nitrogen in agricultural soils-agronomy monograph no. 22. American Society of Agronomy and Soil Science Society of America, Madison, pp 651–688
Stanford JA, Lorang MS, Hauer FR (2005) Plenary lecture: the shifting habitat mosaic of river ecosystems. Verh Int Ver Theor Angew Limnol 29:123–136
Stevenson FJ, Cole MA (1999) The internal cycle of nitrogen in soil, chapter 6. In: Cycles of soil: carbon, nitrogen, phosphorus, sulfur, micronutrients. Wiley, New York
USGS Gage 12358500. United States Geological Survey 2010 Retrieved on May 14, 2010. Gauge located on Middle Fork of the Flathead River 1.3 miles west of West Glacier, MT. http://water.usgs.gov/
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biochemistry 13:87–115
Vitousek PM, Walker LR (1989) Biological invasion by Myrica faya in Hawai’i: plant demography, nitrogen fixation, ecosystem effects. Ecol Monogr 59:247–265
Walker LR (1989) Soil nitrogen changes during primary succession on a floodplain in Alaska, USA. Arct Alp Res 21:341–349
Walker LR, Chapin FS III (1986) Physiological controls over seedling growth in primary succession on an Alaskan floodplain. Ecology 67:1508–1523
Walker LR, del Moral R (2003) Primary succession and ecosystem rehabilitation. Cambridge University Press, Cambridge
Weinhold BJ (2007) Comparison of laboratory methods and an in situ method for estimating nitrogen mineralization in an irrigated silt-loam soil. Commun Soil Sci Plant Anal 38:1721–1732
Whited DC, Lorang MS, Harner MJ, Hauer FR, Kimball JS, Stanford JA (2007) Climate, hydrological disturbance, and succession: drivers of floodplain pattern. Ecology 88:940–953
Acknowledgments
We thank the John Dalimata family for access to the Nyack Flood Plain, Art Mckee for coordinating the REU Program and providing advice on forestry research, and the rest of the staff of the Flathead Lake Biological Station for logistical support. We thank Polly Gibson and Chad Backsen for assistance with field work and Ashley Helton for constructive comments on the manuscript. This study was supported by the National Science Foundation REU award (DBI-0353520), NSF Microbial Observatory award (MCB-0348773) and the Gordon and Betty Moore Foundation. Morris also received fellowship funding from the Montana NSF EPSCoR Large River Ecosystems Program (EPS-0701906).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, M.R., Brouwer, B.O., Caves, J.K. et al. Successional changes in soil and hyporheic nitrogen fertility on an alluvial flood plain: implications for riparian vegetation. Aquat. Sci. 72, 519–532 (2010). https://doi.org/10.1007/s00027-010-0153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-010-0153-8