Abstract
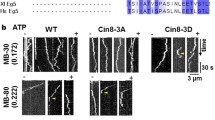
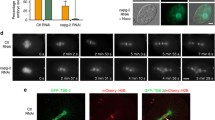
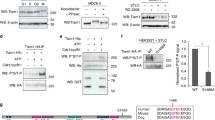
The bipolar kinesin-5 motors perform essential functions in mitotic spindle dynamics. We previously demonstrated that phosphorylation of at least one of the Cdk1 sites in the catalytic domain of the Saccharomyces cerevisiae kinesin-5 Cin8 (S277, T285, S493) regulates its localization to the anaphase spindle. The contribution of these three sites to phospho-regulation of Cin8, as well as the timing of such contributions, remains unknown. Here, we examined the function and spindle localization of phospho-deficient (serine/threonine to alanine) and phospho-mimic (serine/threonine to aspartic acid) Cin8 mutants. In vitro, the three Cdk1 sites undergo phosphorylation by Clb2-Cdk1. In cells, phosphorylation of Cin8 affects two aspects of its localization to the anaphase spindle, translocation from the spindle-pole bodies (SPBs) region to spindle microtubules (MTs) and the midzone, and detachment from the mitotic spindle. We found that phosphorylation of S277 is essential for the translocation of Cin8 from SPBs to spindle MTs and the subsequent detachment from the spindle. Phosphorylation of T285 mainly affects the detachment of Cin8 from spindle MTs during anaphase, while phosphorylation at S493 affects both the translocation of Cin8 from SPBs to the spindle and detachment from the spindle. Only S493 phosphorylation affected the anaphase spindle elongation rate. We conclude that each phosphorylation site plays a unique role in regulating Cin8 functions and postulate a model in which the timing and extent of phosphorylation of the three sites orchestrates the anaphase function of Cin8.








Similar content being viewed by others
References
Maddox P, Straight A, Coughlin P, Mitchison TJ, Salmon ED (2003) Direct observation of microtubule dynamics at kinetochores in Xenopus extract spindles: implications for spindle mechanics. J Cell Biol 162(3):377–382
Hoyt MA, He L, Loo KK, Saunders WS (1992) Two Saccharomyces cerevisiae kinesin-related gene products required for mitotic spindle assembly. J Cell Biol 118(1):109–120
Hagan I, Yanagida M (1992) Kinesin-related cut7 protein associates with mitotic and meiotic spindles in fission yeast. Nature 356(6364):74–76. doi:10.1038/356074a0
Heck MM, Pereira A, Pesavento P, Yannoni Y, Spradling AC, Goldstein LS (1993) The kinesin-like protein KLP61F is essential for mitosis in Drosophila. J Cell Biol 123(3):665–679
Blangy A, Lane HA, d’Herin P, Harper M, Kress M, Nigg EA (1995) Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83(7):1159–1169
Goshima G, Vale RD (2005) Cell cycle-dependent dynamics and regulation of mitotic kinesins in Drosophila S2 cells. Mol Biol Cell 16(8):3896–3907
Scholey JE, Nithianantham S, Scholey JM, Al-Bassam J (2014) Structural basis for the assembly of the mitotic motor Kinesin-5 into bipolar tetramers. Elife 3:e02217. doi:10.7554/eLife.02217
Hildebrandt ER, Gheber L, Kingsbury T, Hoyt MA (2006) Homotetrameric form of Cin8p, a Saccharomyces cerevisiae kinesin-5 motor, is essential for its in vivo function. J Biol Chem 281(36):26004–26013
Gordon DM, Roof DM (1999) The kinesin-related protein Kip1p of Saccharomyces cerevisiae is bipolar. J Biol Chem 274(40):28779–28786
Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM (1996) A bipolar kinesin. Nature 379(6562):270–272
Gheber L, Kuo SC, Hoyt MA (1999) Motile properties of the kinesin-related Cin8p spindle motor extracted from Saccharomyces cerevisiae cells. J Biol Chem 274(14):9564–9572
Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF (2005) The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435(7038):114–118
Roof DM, Meluh PB, Rose MD (1992) Kinesin-related proteins required for assembly of the mitotic spindle. J Cell Biol 118:95–108
Saunders WS, Hoyt MA (1992) Kinesin-related proteins required for structural integrity of the mitotic spindle. Cell 70(3):451–458
Saunders WS, Koshland D, Eshel D, Gibbons IR, Hoyt MA (1995) Saccharomyces cerevisiae kinesin- and dynein-related proteins required for anaphase chromosome segregation. J Cell Biol 128(4):617–624
Gerson-Gurwitz A, Movshovich N, Avunie R, Fridman V, Moyal K, Katz B, Hoyt MA, Gheber L (2009) Mid-anaphase arrest in S. cerevisiae cells eliminated for the function of Cin8 and dynein. Cell Mol Life Sci 66(2):301–313
Movshovich N, Fridman V, Gerson-Gurwitz A, Shumacher I, Gertsberg I, Fich A, Hoyt MA, Katz B, Gheber L (2008) Slk19-dependent mid-anaphase pause in kinesin-5-mutated cells. J Cell Sci 121(15):2529–2539
Straight AF, Sedat JW, Murray AW (1998) Time-lapse microscopy reveals unique roles for kinesins during anaphase in budding yeast. J Cell Biol 143(3):687–694
Fridman V, Gerson-Gurwitz A, Movshovich N, Kupiec M, Gheber L (2009) Midzone organization restricts interpolar microtubule plus-end dynamics during spindle elongation. EMBO Rep 10(4):387–393
Gardner MK, Bouck DC, Paliulis LV, Meehl JB, O’Toole ET, Haase J, Soubry A, Joglekar AP, Winey M, Salmon ED, Bloom K, Odde DJ (2008) Chromosome congression by Kinesin-5 motor-mediated disassembly of longer kinetochore microtubules. Cell 135(5):894–906
Wargacki MM, Tay JC, Muller EG, Asbury CL, Davis TN (2010) Kip3, the yeast kinesin-8, is required for clustering of kinetochores at metaphase. Cell Cycle 9(13):2581–2588
Tytell JD, Sorger PK (2006) Analysis of kinesin motor function at budding yeast kinetochores. JCB 172(6):861–874
Gibbs KL, Greensmith L, Schiavo G (2015) Regulation of axonal transport by protein kinases. Trends Biochem Sci 40(10):597–610. doi:10.1016/j.tibs.2015.08.003
Morfini G, Schmidt N, Weissmann C, Pigino G, Kins S (2016) Conventional kinesin: biochemical heterogeneity and functional implications in health and disease. Brain Res Bull. doi:10.1016/j.brainresbull.2016.06.009
Ritter A, Kreis NN, Louwen F, Wordeman L, Yuan J (2015) Molecular insight into the regulation and function of MCAK. Crit Rev Biochem Mol Biol 51(4):228–245. doi:10.1080/10409238.2016.1178705
Waitzman JS, Rice SE (2014) Mechanism and regulation of kinesin-5, an essential motor for the mitotic spindle. Biol Cell 106(1):1–12. doi:10.1111/boc.201300054
Wojcik EJ, Buckley RS, Richard J, Liu L, Huckaba TM, Kim S (2013) Kinesin-5: cross-bridging mechanism to targeted clinical therapy. Gene 531(2):133–149. doi:10.1016/j.gene.2013.08.004
Manser C, Guillot F, Vagnoni A, Davies J, Lau KF, McLoughlin DM, De Vos KJ, Miller CC (2012) Lemur tyrosine kinase-2 signalling regulates kinesin-1 light chain-2 phosphorylation and binding of Smad2 cargo. Oncogene 31(22):2773–2782. doi:10.1038/onc.2011.437
Craige B, Witman GB (2014) Flipping a phosphate switch on kinesin-II to turn IFT around. Dev Cell 30(5):492–493. doi:10.1016/j.devcel.2014.08.019
Fesquet D, De Bettignies G, Bellis M, Espeut J, Devault A (2015) Binding of Kif23-iso1/CHO1 to 14-3-3 is regulated by sequential phosphorylations at two LATS kinase consensus sites. PLoS One 10(2):e0117857. doi:10.1371/journal.pone.0117857
Ogawa T, Hirokawa N (2015) Microtubule destabilizer KIF2A undergoes distinct site-specific phosphorylation cascades that differentially affect neuronal morphogenesis. Cell Rep 12(11):1774–1788. doi:10.1016/j.celrep.2015.08.018
Drechsler H, Tan AN, Liakopoulos D (2015) Yeast GSK-3 kinase regulates astral microtubule function through phosphorylation of the microtubule-stabilizing kinesin Kip2. J Cell Sci 128(21):3910–3921. doi:10.1242/jcs.166686
Zong H, Carnes SK, Moe C, Walczak CE, Ems-McClung SC (2016) The far C-terminus of MCAK regulates its conformation and spindle pole focusing. Mol Biol Cell 27(9):1451–1464. doi:10.1091/mbc.E15-10-0699
Cantuti Castelvetri L, Givogri MI, Hebert A, Smith B, Song Y, Kaminska A, Lopez-Rosas A, Morfini G, Pigino G, Sands M, Brady ST, Bongarzone ER (2013) The sphingolipid psychosine inhibits fast axonal transport in Krabbe disease by activation of GSK3beta and deregulation of molecular motors. J Neurosci 33(24):10048–10056. doi:10.1523/JNEUROSCI.0217-13.2013
Morfini G, Szebenyi G, Elluru R, Ratner N, Brady ST (2002) Glycogen synthase kinase 3 phosphorylates kinesin light chains and negatively regulates kinesin-based motility. EMBO J 21(3):281–293. doi:10.1093/emboj/21.3.281
Pigino G, Morfini G, Atagi Y, Deshpande A, Yu C, Jungbauer L, LaDu M, Busciglio J, Brady S (2009) Disruption of fast axonal transport is a pathogenic mechanism for intraneuronal amyloid beta. Proc Natl Acad Sci USA 106(14):5907–5912. doi:10.1073/pnas.0901229106
Morfini GA, Bosco DA, Brown H, Gatto R, Kaminska A, Song Y, Molla L, Baker L, Marangoni MN, Berth S, Tavassoli E, Bagnato C, Tiwari A, Hayward LJ, Pigino GF, Watterson DM, Huang CF, Banker G, Brown RH Jr, Brady ST (2013) Inhibition of fast axonal transport by pathogenic SOD1 involves activation of p38 MAP kinase. PLoS One 8(6):e65235. doi:10.1371/journal.pone.0065235
Morfini G, Pigino G, Szebenyi G, You Y, Pollema S, Brady ST (2006) JNK mediates pathogenic effects of polyglutamine-expanded androgen receptor on fast axonal transport. Nat Neurosci 9(7):907–916. doi:10.1038/nn1717
Morfini GA, You YM, Pollema SL, Kaminska A, Liu K, Yoshioka K, Bjorkblom B, Coffey ET, Bagnato C, Han D, Huang CF, Banker G, Pigino G, Brady ST (2009) Pathogenic huntingtin inhibits fast axonal transport by activating JNK3 and phosphorylating kinesin. Nat Neurosci 12(7):864–871. doi:10.1038/nn.2346
Courtheoux T, Gay G, Reyes C, Goldstone S, Gachet Y, Tournier S (2007) Dynein participates in chromosome segregation in fission yeast. Biol Cell 99(11):627–637
Padzik A, Deshpande P, Hollos P, Franker M, Rannikko EH, Cai D, Prus P, Magard M, Westerlund N, Verhey KJ, James P, Hoogenraad CC, Coffey ET (2016) KIF5C S176 phosphorylation regulates microtubule binding and transport efficiency in mammalian neurons. Front Cell Neurosci 10:57. doi:10.3389/fncel.2016.00057
Han X, Adames K, Sykes EM, Srayko M (2015) The KLP-7 residue S546 is a putative aurora kinase site required for microtubule regulation at the centrosome in C. elegans. PLoS One 10(7):e0132593. doi:10.1371/journal.pone.0132593
Andrews PD, Ovechkina Y, Morrice N, Wagenbach M, Duncan K, Wordeman L, Swedlow JR (2004) Aurora B regulates MCAK at the mitotic centromere. Dev Cell 6(2):253–268
Lan W, Zhang X, Kline-Smith SL, Rosasco SE, Barrett-Wilt GA, Shabanowitz J, Hunt DF, Walczak CE, Stukenberg PT (2004) Aurora B phosphorylates centromeric MCAK and regulates its localization and microtubule depolymerization activity. Curr Biol 14(4):273–286
Ohi R, Sapra T, Howard J, Mitchison TJ (2004) Differentiation of cytoplasmic and meiotic spindle assembly MCAK functions by Aurora B-dependent phosphorylation. Mol Biol Cell 15(6):2895–2906. doi:10.1091/mbc.E04-02-0082
Zhang X, Ems-McClung SC, Walczak CE (2008) Aurora A phosphorylates MCAK to control ran-dependent spindle bipolarity. Mol Biol Cell 19(7):2752–2765. doi:10.1091/mbc.E08-02-0198
Mennella V, Tan DY, Buster DW, Asenjo AB, Rath U, Ma A, Sosa HJ, Sharp DJ (2009) Motor domain phosphorylation and regulation of the Drosophila kinesin 13, KLP10A. J Cell Biol 186(4):481–490
Ritter A, Sanhaji M, Friemel A, Roth S, Rolle U, Louwen F, Yuan J (2015) Functional analysis of phosphorylation of the mitotic centromere-associated kinesin by Aurora B kinase in human tumor cells. Cell Cycle 14(23):3755–3767. doi:10.1080/15384101.2015.1068481
Sanhaji M, Friel CT, Kreis NN, Kramer A, Martin C, Howard J, Strebhardt K, Yuan J (2010) Functional and spatial regulation of mitotic centromere-associated kinesin by cyclin-dependent kinase 1. Mol Cell Biol 30(11):2594–2607
Giet R, Uzbekov R, Cubizolles F, Le Guellec K, Prigent C (1999) The Xenopus laevis aurora-related protein kinase pEg2 associates with and phosphorylates the kinesin-related protein XlEg5. J Biol Chem 274(21):15005–15013
Sharp DJ, McDonald KL, Brown HM, Matthies HJ, Walczak C, Vale RD, Mitchison TJ, Scholey JM (1999) The bipolar kinesin, KLP61F, cross-links microtubules within interpolar microtubule bundles of Drosophila embryonic mitotic spindles. J Cell Biol 144(1):125–138
Sawin KE, Mitchison TJ (1995) Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc Natl Acad Sci USA 92(10):4289–4293
Cahu J, Olichon A, Hentrich C, Schek H, Drinjakovic J, Zhang C, Doherty-Kirby A, Lajoie G, Surrey T (2008) Phosphorylation by Cdk1 increases the binding of Eg5 to microtubules in vitro and in Xenopus egg extract spindles. PLoS One 3(12):e3936
Bishop JD, Han Z, Schumacher JM (2005) The Caenorhabditis elegans Aurora B kinase AIR-2 phosphorylates and is required for the localization of a BimC kinesin to meiotic and mitotic spindles. Mol Biol Cell 16(2):742–756
Drummond DR, Hagan IM (1998) Mutations in the bimC box of Cut7 indicate divergence of regulation within the bimC family of kinesin related proteins. J Cell Sci 111(Pt 7):853–865
Avunie-Masala R, Movshovich N, Nissenkorn Y, Gerson-Gurwitz A, Fridman V, Koivomagi M, Loog M, Hoyt MA, Zaritsky A, Gheber L (2011) Phospho-regulation of kinesin-5 during anaphase spindle elongation. J Cell Sci 124(Pt 6):873–878
Olsen JV, Vermeulen M, Santamaria A, Kumar C, Miller ML, Jensen LJ, Gnad F, Cox J, Jensen TS, Nigg EA, Brunak S, Mann M (2010) Quantitative phosphoproteomics reveals widespread full phosphorylation site occupancy during mitosis. Sci Signal 3(104):ra3. doi:10.1126/scisignal.2000475
Smith E, Hegarat N, Vesely C, Roseboom I, Larch C, Streicher H, Straatman K, Flynn H, Skehel M, Hirota T, Kuriyama R, Hochegger H (2011) Differential control of Eg5-dependent centrosome separation by Plk1 and Cdk1. EMBO J 30(11):2233–2245. doi:10.1038/emboj.2011.120
Kahn OI, Sharma V, Gonzalez-Billault C, Baas PW (2015) Effects of kinesin-5 inhibition on dendritic architecture and microtubule organization. Mol Biol Cell 26(1):66–77. doi:10.1091/mbc.E14-08-1313
Garcia K, Stumpff J, Duncan T, Su TT (2009) Tyrosines in the kinesin-5 head domain are necessary for phosphorylation by Wee1 and for mitotic spindle integrity. Curr Biol 19(19):1670–1676
Chee MK, Haase SB (2010) B-cyclin/CDKs regulate mitotic spindle assembly by phosphorylating kinesins-5 in budding yeast. PLoS Genet 6:e1000935
Shapira O, Gheber L (2016) Motile properties of the bi-directional kinesin-5 Cin8 are affected by phosphorylation in its motor domain. Sci Rep 6(25597):25597
Schuyler SC, Liu JY, Pellman D (2003) The molecular function of Ase1p: evidence for a MAP-dependent midzone-specific spindle matrix. Microtubule-associated proteins. J Cell Biol 160(4):517–528
Khmelinskii A, Roostalu J, Roque H, Antony C, Schiebel E (2009) Phosphorylation-dependent protein interactions at the spindle midzone mediate cell cycle regulation of spindle elongation. Dev Cell 17(2):244–256
Kull FJ, Sablin EP, Lau R, Fletterick RJ, Vale RD (1996) Crystal structure of the kinesin motor domain reveals a structural similarity to myosin. Nature 380(6574):550–555
Sablin EP, Kull FJ, Cooke R, Vale RD, Fletterick RJ (1996) Crystal structure of the motor domain of the kinesin-related motor ncd. Nature 380(6574):555–559
Roostalu J, Hentrich C, Bieling P, Telley IA, Schiebel E, Surrey T (2011) Directional switching of the Kinesin cin8 through motor coupling. Science 332(6025):94–99
Gerson-Gurwitz A, Thiede C, Movshovich N, Fridman V, Podolskaya M, Danieli T, Lakamper S, Klopfenstein DR, Schmidt CF, Gheber L (2011) Directionality of individual kinesin-5 Cin8 motors is modulated by loop 8, ionic strength and microtubule geometry. EMBO J 30(24):4942–4954
Shapira O, Goldstein A, Al-Bassam J, Gheber L (2017) A potential physiological role for bi-directional motility and motor clustering of mitotic kinesin-5 Cin8 in yeast mitosis. J Cell Sci 130(4):725–734
Konig C, Maekawa H, Schiebel E (2010) Mutual regulation of cyclin-dependent kinase and the mitotic exit network. J Cell Biol 188(3):351–368
Fridman V, Gerson-Gurwitz A, Shapira O, Movshovich N, Lakamper S, Schmidt CF, Gheber L (2013) Kinesin-5 Kip1 is a bi-directional motor that stabilizes microtubules and tracks their plus-ends in vivo. J Cell Sci 126(Pt 18):4147–4159. doi:10.1242/jcs.125153
Hildebrandt ER, Hoyt MA (2001) Cell cycle-dependent degradation of the Saccharomyces cerevisiae spindle motor Cin8p requires APC(Cdh1) and a bipartite destruction sequence. Mol Biol Cell 12(11):3402–3416
Diogo V, Teixeira J, Silva PM, Bousbaa H (2016) Spindle assembly checkpoint as a potential target in colorectal cancer: current status and future perspectives. Clin Colorectal Cancer 23(16):30080–30089
Musacchio A (2015) The molecular biology of spindle assembly checkpoint signaling dynamics. Curr Biol 25(20):R1002–R1018
Topham CH, Taylor SS (2013) Mitosis and apoptosis: how is the balance set? Curr Opin Cell Biol 25(6):780–785. doi:10.1016/j.ceb.2013.07.003
Bardin AJ, Amon A (2001) Men and sin: what’s the difference? Nat Rev Mol Cell Biol 2(11):815–826. doi:10.1038/35099020
Hotz M, Leisner C, Chen D, Manatschal C, Wegleiter T, Ouellet J, Lindstrom D, Gottschling DE, Vogel J, Barral Y (2012) Spindle pole bodies exploit the mitotic exit network in metaphase to drive their age-dependent segregation. Cell 148(5):958–972
Segal M (2011) Mitotic exit control: a space and time odyssey. Curr Biol 21(20):R857–R859. doi:10.1016/j.cub.2011.09.023
Gigant B, Wang W, Dreier B, Jiang Q, Pecqueur L, Pluckthun A, Wang C, Knossow M (2013) Structure of a kinesin-tubulin complex and implications for kinesin motility. Nat Struct Mol Biol 20(8):1001–1007
Zhang X, Lan W, Ems-McClung SC, Stukenberg PT, Walczak CE (2007) Aurora B phosphorylates multiple sites on mitotic centromere-associated kinesin to spatially and temporally regulate its function. Mol Biol Cell 18(9):3264–3276. doi:10.1091/mbc.E07-01-0086
Duselder A, Fridman V, Thiede C, Wiesbaum A, Goldstein A, Klopfenstein DR, Zaitseva O, Janson ME, Gheber L, Schmidt CF (2015) Deletion of the tail domain of the kinesin-5 Cin8 affects its directionality. J Biol Chem 19:620799
Thiede C, Fridman V, Gerson-Gurwitz A, Gheber L, Schmidt CF (2012) Regulation of bi-directional movement of single kinesin-5 Cin8 molecules. Bioarchitecture 2(2):70–74
Queralt E, Lehane C, Novak B, Uhlmann F (2006) Downregulation of PP2A(Cdc55) phosphatase by separase initiates mitotic exit in budding yeast. Cell 125(4):719–732
Chiroli E, Rancati G, Catusi I, Lucchini G, Piatti S (2009) Cdc14 inhibition by the spindle assembly checkpoint prevents unscheduled centrosome separation in budding yeast. Mol Biol Cell 20(10):2626–2637
Roccuzzo M, Visintin C, Tili F, Visintin R (2015) FEAR-mediated activation of Cdc14 is the limiting step for spindle elongation and anaphase progression. Nat Cell Biol 17(3):251–261. doi:10.1038/ncb3105
Ross KE, Cohen-Fix O (2004) A role for the FEAR pathway in nuclear positioning during anaphase. Dev Cell 6(5):729–735
D’Amours D, Amon A (2004) At the interface between signaling and executing anaphase—Cdc14 and the FEAR network. Genes Dev 18(21):2581–2595
Yellman CM, Roeder GS (2015) Cdc14 early anaphase release, FEAR, is limited to the nucleus and dispensable for efficient mitotic exit. PLoS One 10(6):e0128604
Stegmeier F, Visintin R, Amon A (2002) Separase, polo kinase, the kinetochore protein Slk19, and Spo12 function in a network that controls Cdc14 localization during early anaphase. Cell 108(2):207–220
Pellman D, Bagget M, Tu YH, Fink GR, Tu H (1995) Two microtubule-associated proteins required for anaphase spindle movement in Saccharomyces cerevisiae. J Cell Biol 130(6):1373–1385
Fu C, Ward JJ, Loiodice I, Velve-Casquillas G, Nedelec FJ, Tran PT (2009) Phospho-regulated interaction between kinesin-6 Klp9p and microtubule bundler Ase1p promotes spindle elongation. Dev Cell 17(2):257–267
Rozelle DK, Hansen SD, Kaplan KB (2011) Chromosome passenger complexes control anaphase duration and spindle elongation via a kinesin-5 brake. J Cell Biol 193(2):285–294
Saunders AM, Powers J, Strome S, Saxton WM (2007) Kinesin-5 acts as a brake in anaphase spindle elongation. Curr Biol 17(12):R453–R454
Shimamoto Y, Forth S, Kapoor TM (2015) Measuring pushing and braking forces generated by ensembles of kinesin-5 crosslinking two microtubules. Dev Cell 34(6):669–681
Cottingham FR, Gheber L, Miller DL, Hoyt MA (1999) Novel roles for Saccharomyces cerevisiae mitotic spindle motors. J Cell Biol 147(2):335–350
Acknowledgements
We thank Vladimir Fridman, Yael Nissenkorn and Maria Podolskaya from the LG laboratory for providing plasmids for this study. We thank Liam Holt, NYU, for critical reading of this manuscript and fruitful discussions, and Ken Kaplan, UC Davis, for fruitful discussions. This work was supported by the Israel Science Foundation grant number 165/13, awarded to LG; the ERC Consolidator Grant 649124 and a Grant from Estonian Research Council IUT2-21 awarded to ML.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Goldstein, A., Siegler, N., Goldman, D. et al. Three Cdk1 sites in the kinesin-5 Cin8 catalytic domain coordinate motor localization and activity during anaphase. Cell. Mol. Life Sci. 74, 3395–3412 (2017). https://doi.org/10.1007/s00018-017-2523-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2523-z