Abstract
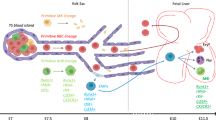
Two different models describe the development of definitive hematopoiesis and hematopoietic stem cells (HSCs). In one of these, the visceral yolk sac serves as a starting point of relatively lengthy developmental process culminating in the fetal liver hematopoiesis. In another, the origin of adult hematopoiesis is split between the yolk sac and the dorsal aorta, which has a peculiar capacity to generate definitive HSCs. Despite a large amount of experimental data consistent with the latter view, it becomes increasingly unsustainable in the light of recent cell tracing studies. Moreover, analysis of the published studies supporting the aorta-centered version uncovers significant caveats in standard experimental approach and argumentation. As a result, the theory cannot offer feasible cellular mechanisms of the HSC emergence. This review summarizes key efforts to discern the developmental pathway of the adult-type HSCs and attempts to put forward a hypothesis on the inflammatory mechanisms of hematopoietic ontogenesis.




Similar content being viewed by others
References
Medvinsky A, Rybtsov S, Taoudi S (2011) Embryonic origin of the adult hematopoietic system: advances and questions. Development 138:1017–1031
Lensch MW, Daley GQ (2004) Origins of mammalian hematopoiesis: in vivo paradigms and in vitro models. Curr Top Dev Biol 60:127–196
Moore MAS, Metcalf D (1970) Ontogeny of the haemopoietic system: yolk sac origin of in vivo and in vitro colony-forming cells in the developing mouse embryo. Br J Haematol 18:279–296
Metcalf D, Moore MAS (1971) Haemopoietic cells: North-Holland research monographs. Front Biol 24:130
Moore MAS, Owen JJT (1967) Stem-cell migration in developing myeloid and lymphoid systems. Lancet 2:658–659
Müller AM, Medvinsky AL, Strouboulis J, Grosveld F, Dzierzak E (1994) Development of hematopoietic stem cell activity in the mouse embryo. Immunity 1:291–301
Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA (2011) Erythroid/myeloid progenitors and hematopoietic stem cells originate from distinct populations of endothelial cells. Cell Stem Cell 9:541–552
Fitch SR, Kimber GM, Wilson NK, Parker A, Mirshekar-Syahkal B, Göttgens B, Medvinsky A, Dzierzak E, Ottersbach K (2012) Signalling from the sympathetic nervous system regulates hematopoietic stem cell emergence during embryogenesis. Cell Stem Cell 11:554–566
Li Z, Lan Y, He W, Chen D, Wang J, Zhou F, Wang Y, Sun H, Chen X, Xu C, Li S, Pang Y, Zhang G, Yang L, Zhu L, Fan M, Shang A, Ju Z, Luo L, Ding Y, Guo W, Yuan W, Yang X, Liu B (2012) Mouse embryonic head as a site for hematopoietic stem cell development. Cell Stem Cell 11:663–675
Medvinsky A, Dzierzak E (1996) Definitive hematopoiesis is autonomously initiated by the AGM region. Cell 86:897–906
de Bruijn M, Speck NA, Peeters MC, Dzierzak E (2000) Definitive hematopoietic stem cells first develop within the major arterial regions of the mouse embryo. EMBO J 19:2465–2474
Gekas C, Dieterlen-Lièvre F, Orkin SH, Mikkola HKA (2005) The placenta is a niche for hematopoietic stem cells. Dev Cell 8:365–375
Ottersbach K, Dzierzak E (2005) The murine placenta contains hematopoietic stem cells within the vascular labyrinth region. Dev Cell 8:377–387
Alberts B, Bray D, Lewis J, Raff M, Roberts K, Watson JD (1994) Cell diversification in the early animal embryo. In: molecular biology of the cell, 3rd edn. Garland Science, New York
Zernicka-Goetz M (2005) Cleavage pattern and emerging asymmetry of the mouse embryo. Nat Rev Mol Cell Biol 6:919–928
Nichols J, Smith A (2009) Naive and primed pluripotent states. Cell Stem Cell 4:487–492
Belaoussoff M, Farrington SM, Baron MH (1998) Hematopoietic induction and respecification of A-P identity by visceral endoderm signaling in the mouse embryo. Development 125:5009–5018
Dyer MA, Farrington SM, Mohn D, Munday JR, Baron MH (2001) Indian hedgehog activates hematopoiesis and vasculogenesis and can respecify prospective neuroectodermal cell fate in the mouse embryo. Development 128:1717–1730
Murakami Y, Hirata H, Miyamoto Y, Nagahashi A, Sawa Y, Jakt M, Asahara T, Kawamata S (2007) Isolation of cardiac cells from E8.5 yolk sac by ALCAM (CD166) expression. Mech Dev 124:830–839
Van Handel B, Montel-Hagen A, Sasidharan R, Nakano H, Ferrari R, Boogerd CJ, Schredelseker J, Wang Y, Hunter S, Org T, Zhou J, Li X, Pellegrini M, Chen J-N, Orkin SH, Kurdistani SK, Evans SM, Nakano A, Mikkola HKA (2012) Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150:590–605
Morgan HD, Santos F, Green K, Dean W, Reik W (2005) Epigenetic reprogramming in mammals. Hum Mol Genet 14:R47–R58
De Angelis L, Berghella L, Coletta M, Lattanzi L, Zanchi M, Cusella-De Angelis MG, Ponzetto C, Cossu G (1999) Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration. J Cell Biol 147:869–877
Tajbakhsh S, Vivarelli E, Cusella-De Angelis G, Rocancourt D, Buckingham M, Cossu G (1994) A population of myogenic cells derived from the mouse neural tube. Neuron 13:813–821
Palis J, Robertson S, Kennedy M, Wall C, Keller G (1999) Development of erythroid and myeloid progenitors in the yolk sac and embryo proper of the mouse. Development 126:5073–5081
Scott EW, Simon MC, Anastasi J, Singh H (1994) Requirement of transcription factor PU.1 in the development of multiple hematopoietic lineages. Science 265:1573–1577
Scott EW, Fisher RC, Olson MC, Kehrli EW, Simon MC, Singh H (1997) PU.1 functions in a cell-autonomous manner to control the differentiation of multipotential lymphoid-myeloid progenitors. Immunity 6:437–447
Anderson KL, Smith KA, Conners K, McKercher SR, Maki RA, Torbett BE (1998) Myeloid development is selectively disrupted in PU.1 null mice. Blood 91:3702–3710
Fisher RC, Scott EW (1998) Role of PU.1 in hematopoiesis. Stem cells 16:25–37
Lichanska AM, Browne CM, Henkel GW, Murphy KM, Ostrowski MC, McKercher SR, Maki RA, Hume DA (1999) Differentiation of the mononuclear phagocyte system during mouse embryogenesis: the role of transcription factor PU.1. Blood 94:127–138
Robin C, Ottersbach K, Durand C, Peeters M, Vanes L, Tybulewicz V, Dzierzak E (2006) An unexpected role for IL-3 in the embryonic development of hematopoietic stem cells. Dev Cell 11:171–180
Ogawa M, Nishikawa S, Ikuta K, Yamamura F, Naito M, Takahashi K, Nishikawa S-I (1988) B cell ontogeny in murine embryo studied by a culture system with the monolayer of a stromal cell clone, ST2: B cell progenitor develops first in the embryonal body rather than in the yolk sac. EMBO J 7:1337–1343
Palacios R, Imhof BA (1993) At day 8–8.5 of mouse development the yolk sac, not the embryo proper, has lymphoid precursor potential in vivo and in vitro. Proc Natl Acad Sci USA 90:6581–6585
Cumano A, Dieterlen-Lievre F, Godin I (1996) Lymphoid potential, probed before circulation in mouse, is restricted to caudal intraembryonic splanchnopleura. Cell 86:907–916
Tanaka Y, Hayashi M, Kubota Y, Nagai H, Sheng G, Nishikawa S-I, Samokhvalov IM (2012) Early ontogenic origin of the hematopoietic stem cell lineage. Proc Natl Acad Sci USA 109:4515–4520
Collins LS, Dorshkind K (1987) A stromal cell line from myeloid long-term bone marrow cultures can support myelopoiesis and B lymphopoiesis. J Immunol 138:1082–1087
Nakano T, Kodama H, Honjo T (1994) Generation of lymphohematopoietic cells from embryonic stem cells in culture. Science 265:1098–1101
Gofflot F, Hall M, Morriss-Kay GM (1997) Genetic patterning of the developing mouse tail at the time of posterior neuropore closure. Dev Dyn 210:431–445
Yoshimoto M, Porayette P, Yoder MC (2008) Overcoming obstacles in the search for the site of hematopoietic stem cell emergence. Cell Stem Cell 3:583–586
Tabata H, Nakajima K (2008) Labeling embryonic mouse central nervous system cells by in utero electroporation. Dev Growth Differ 50:507–511
Martin CS, Moriyama A, Zon LI (2011) Hematopoietic stem cells, hematopoiesis and disease: lessons from the zebrafish model. Genome Med 3:83
Liu F, Walmsley M, Rodaway A, Patient R (2008) Fli1 acts at the top of the transcriptional network driving blood and endothelial development. Curr Biol 18:1234–1240
Stern CD, Downs KM (2012) The hypoblast (visceral endoderm): an evo-devo perspective. Development 139:1059–1069
Sood R, Liu P (2012) Novel insights into the genetic controls of primitive and definitive hematopoiesis from zebrafish models. Adv Hematol 2012:830703
Palis J, Yoder MC (2001) Yolk-sac hematopoiesis: the first blood cells of mouse and man. Exp Hematol 29:927–936
Brotherton TW, Chui DH, Gualdie J, Patterson M (1979) Hemoglobin ontogeny during normal mouse fetal development. Proc Natl Acad Sci USA 76:2853–2857
Kaufman MH (1992) The atlas of mouse development. Academic Press, San Diego, p 128
Kingsley PD, Malik J, Fantauzzo KA, Palis J (2004) Yolk sac-derived primitive erythroblasts enucleate during mammalian embryogenesis. Blood 104:19–25
Sabin FR (1920) Studies on the origin of blood vessels and of red corpuscles as seen in the living blastoderm of the chick during the second day of incubation. Carnegie Inst Wash Publ Contrib Embryol 9:213–262
Murray P (1932) The development in vitro of the blood of early chick embryo. Proc Royal Soc London 111:497–521
Kennedy M, Firpo M, Choi K, Wall C, Robertson S, Kabrun N, Keller G (1997) A common precursor for primitive erythropoiesis and definitive haematopoiesis. Nature 386:488–493
Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G (1998) A common precursor for hematopoietic and endothelial. Development 125:725–732
Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Valerie K (2003) Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development 130:4217–4227
Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G (2004) Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature 432:625–630
Ema M, Faloon P, Zhang WJ, Hirashima M, Reid T, Stanford WL, Orkin S, Choi K, Rossant J (2003) Combinatorial effects of Flk1 and Tal1 on vascular and hematopoietic development in the mouse. Genes Dev 17:380–393
Lancrin C, Sroczynska P, Stephenson C, Allen T, Kouskoff V, Lacaud G (2009) The haemangioblast generates haematopoietic cells through a haemogenic endothelium stage. Nature 457:892–895
Ferkowicz MJ, Starr M, Xie X, Li W, Johnson SA, Shelley WC, Morrison PR, Yoder MC (2003) CD41 expression defines the onset of primitive and definitive hematopoiesis in the murine embryo. Development 130:4393–4403
Ema M, Yokomizo T, Wakamatsu A, Terunuma T, Yamamoto M, Takahashi S (2006) Primitive erythropoiesis from mesodermal precursors expressing VE-cadherin, PECAM-1, Tie2, endoglin, and CD34 in the mouse embryo. Blood 108:4018–4024
Ueno H, Weissman IL (2006) Clonal analysis of mouse development reveals a polyclonal origin for yolk sac blood islands. Dev Cell 11:519–533
Samokhvalov IM, Samokhvalova NI, Nishikawa S-I (2007) Cell tracing shows the contribution of the yolk sac to adult haematopoiesis. Nature 446:1056–1061
Ferkowicz MJ, Yoder MC (2005) Blood island formation: longstanding observations and modern interpretations. Exp Hematol 33:1041–1047
Minasi MG, Riminucci M, De Angelis L, Borello U, Berarducci B, Innocenzi A, Caprioli A, Sirabella D, Baiocchi M, De Maria R, Boratto R, Jaffredo T, Broccoli V, Bianco P, Cossu G (2002) The meso-angioblast: a multipotent, self-renewing cell that originates from the dorsal aorta and differentiates into most mesodermal tissues. Development 129:2773–2783
Samokhvalov IM (2012) A long way to stemness. Cell Cycle 11:2965–2966
Pimanda JE, Ottersbach K, Knezevic K, Kinston S, Chan WYI, Wilson NK, Landry J-R, Wood AD, Kolb-Kokocinski A, Green AR, Tannahill D, Lacaud G, Kouskoff V, Göttgens B (2007) Gata2, Fli1, and Scl form a recursively wired gene-regulatory circuit during early hematopoietic development. Proc Natl Acad Sci USA 104:17692–17697
Okuda T, Van Deursen J, Hiebert SW, Grosveld G, Downing JR (1996) AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84:321–330
Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T (2000) A role for hematopoietic stem cells in promoting angiogenesis. Cell 102:199–209
Hart A, Melet F, Grossfeld P, Chien K, Jones C, Tunnacliffe A, Favier R, Bernstein A (2000) Fli-1 is required for murine vascular and megakaryocytic development and is hemizygously deleted in patients with thrombocytopenia. Immunity 13:167–177
Bader BL, Rayburn H, Crowley D, Hynes RO (1998) Extensive vasculogenesis, angiogenesis and organogenesis precede lethality in mice lacking all αv integrins. Cell 95:507–519
Xia L, Ju T, Westmuckett A, An G, Ivanciu L, McDaniel JM, Lupu F, Cummings RD, McEver RP (2004) Defective angiogenesis and fatal embryonic hemorrhage in mice lacking core 1-derived O-glycans. J Cell Biol 164:451–459
Puri MC, Rossant J, Alitalo K, Bernstein A, Partanen J (1995) The receptor tyrosine kinase TIE is required for integrity and survival of vascular endothelial cells. EMBO J 14:5884–5891
Liakhovitskaia A, Gribi R, Stamateris E, Villain G, Jaffredo T, Wilkie R, Gilchrist D, Yang J, Ure J, Medvinsky A (2009) Restoration of Runx1 expression in the Tie2 cell compartment rescues definitive hematopoietic stem cells and extends life of Runx1 knockout animals until birth. Stem Cells 27:1616–1624
Li Z, Chen MJ, Stacy T, Speck NA (2006) Runx1 function in hematopoiesis is required in cells that express Tek. Blood 107:106–110
Yoder MC, Hiatt K, Mukherjee P (1997) In vivo repopulating hematopoietic stem cells are present in the murine yolk sac at day 9.0 postcoitus. Proc Natl Acad Sci USA 94:6776–6780
Yoder MC, Hiatt K, Dutt P, Mukherjee P, Bodine DM, Orlic D (1997) Characterization of definitive lymphohematopoietic stem cells in the day 9 murine yolk sac. Immunity 7:335–344
McGrath KE, Koniski AD, Malik J, Palis J (2003) Circulation is established in a stepwise pattern in the mammalian embryo. Blood 101:1669–1676
Weissman I, Papaioannou V, Gardner R (1978) Fetal hematopoietic origins of the adult hematolymphoid systems. Cold Spring Harbor laboratory Press, New York
Toles JF, Chui DHK, Belbeck LW, Starr E, Barker JE (1989) Hemopoietic stem cells in murine embryonic yolk sac and peripheral blood. Proc Natl Acad Sci USA 86:7456–7459
Lux CT, Yoshimoto M, McGrath K, Conway SJ, Palis J, Yoder MC (2008) All primitive and definitive hematopoietic progenitor cells emerging before E10 in the mouse embryo are products of the yolk sac. Blood 111:3435–3438
Adamo L, Naveiras O, Wenzel PL, McKinney-Freeman S, Mack PJ, Gracio-Sancho J, Suchy-Dicey A, Yoshimoto M, Lensch MW, Yoder MC, Garcia-Cardena G, Daley GQ (2009) Biomechanical forces promote embryonic haematopoiesis. Nature 459:1131–1135
North TE, Goessling W, Peeters M, Li P, Ceol C, Lord AM, Weber GJ, Harris J, Cutting CC, Huang P, Dzierzak E, Zon LI (2009) Hematopoietic stem cells development is dependent on blood flow. Cell 137:736–748
Lancrin C, Mazan M, Stefanska M, Patel R, Lichtinger M, Costa G, Vargel Ö, Wilson NK, Möröy T, Bonifer C, Göttgens B, Kouskoff V, Lacaud G (2012) GFI1 and GFI1B control the loss of endothelial identity of hemogenic endothelium during hematopoietic commitment. Blood 120:314–322
Mukouyama Y, Chiba N, Hara T, Okada H, Ito Y, Kanamaru R, Miyajima A, Satake M, Watanabe T (2000) The AML1 transcription factor function to develop and maintain hematogenic precursor cells in the embryonic aorta-gonad-mesonephros region. Dev Biol 220:27–36
Rhodes KE, Gekas C, Wang Y, Lux CT, Francis CS, Chan DN, Conway S, Orkin SH, Yoder MC, Mikkola HKA (2008) The emergence of hematopoietic stem cells is initiated in the placental vasculature in the absence of circulation. Cell Stem Cell 2:252–263
Cai Z, de Bruijn M, Ma X, Dortland B, Luteijn T, Downing JR, Dzierzak E (2000) Haploinsufficiency of AML1 affects the temporal and spatial generation of hematopoietic stem cells in the mouse embryo. Immunity 13:423–431
Yoder MC (2006) Hematopoietic regulation in the embryo. Haematol Rep 2:86–89
Moore MAS, Owen JJT (1967) Chromosome marker studies in the irradiated chick embryo. Nature 215:1081–1082
Marks PA, Rifkind RA (1972) Protein synthesis: its control in erythropoiesis. Science 175:955–961
Dieterlen-Lièvre F (1975) On the origin of haemopoietic stem cells in the avian embryo: an experimental approach. J Embryol Exp Morphol 33:607–619
Lassila O, Eskola J, Toivanen P, Martin C, Dieterlen-Lievre F (1978) The origin of lymphoid stem cells studied in chick yolk sac-embryo chimaeras. Nature 272:353–354
Dieterlen-Lièvre F, Martin C (1981) Diffuse intraembryonic hemopoiesis in normal and chimeric avian development. Dev Biol 88:180–191
Cormier F, Dieterlen-Lièvre F (1988) The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development 102:272–285
Dzierzak E, Speck NA (2008) Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nat Immunol 9:129–136
Yokomizo T, Dzierzak E (2010) Three-dimensional cartography of hematopoietic clusters in the vasculature of whole mouse embryos. Development 137:3651–3661
Zape JP, Zovein AC (2011) Hemogenic endothelium: origins, regulation, and implications for vascular biology. Semin Cell Dev Biol 22:1036–1047
Cumano A, Ferraz JC, Klaine M, Di Santo JP, Godin I (2001) Intraembryonic, but not yolk sac hematopoietic precursors, isolated before circulation, provide long-term multilineage reconstitution. Immunity 15:477–485
Nishikawa S-I, Nishikawa S, Kawamoto H, Yoshida H, Kizumoto M, Kataoka H, Katsura Y (1998) In vitro generation of lymphohematopoietic cells from endothelial cells purified from murine embryos. Immunity 8:761–769
Nishikawa S-I, Nishikawa S, Hirashima M, Matsuyoshi N, Kodama H (1998) Progressive lineage analysis by cell sorting and culture identifies FLK + VE-cadherin + cells at a diverging point of endothelial and hemopoietic lineages. Development 125:1747–1757
Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA (2009) Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature 457:887–891
Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, Tallquist MD, Iruela-Arispe ML (2008) Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell Stem Cell 3:625–636
Kellendonk C, Tronche F, Casanova E, Anlag K, Opherk C, Schütz G (1999) Inducible site-specific recombination in the brain. J Mol Biol 285:175–182
Kim I, Yilmaz OH, Morrison SJ (2005) CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood 106:903–905
Caveda L, Martin-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E (1996) Inhibition of cultured cell growth by vascular endothelial cadherin (cadherin-5/VE-cadherin). J Clin Invest 98:886–893
Dejana E, Bazzoni G, Lampugnani MG (1999) Vascular endothelial (VE)-cadherin: only an intercellular glue? Exp Cell Res 252:13–19
Carmeliet P, Lampugnani M-G, Moons L, Breviario F, Compernolle V, Bono F, Balconi G, Spagnuolo R, Oosthuyse B, Dewerchin M, Zanetti A, Angellilo A, Mattot V, Nuyens D, Lutgens E, Clotman F, de Ruiter MC, Gittenberger-de Groot A, Poelmann R, Lupu F, Herbert J-M, Collen D, Dejana E (1999) Targeted deficiency or cytosolic truncation of the VE-cadherin gene in mice impairs VEGF-mediated endothelial survival and angiogenesis. Cell 98:147–157
Gory-Fauré S, Prandini M-H, Pointu H, Roullot V, Pignot-Paintrand I, Vernet M, Huber P (1999) Role of vascular endothelial-cadherin in vascular morphogenesis. Development 126:2093–2102
Spratt NT, Haas H (1960) Integrative mechanisms in development of the early chick blastoderm. I. Regulative potentiality of separated parts. J Exp Zool 145:97–137
Taoudi S, Medvinsky A (2007) Functional identification of the hematopoietic stem cell niche in the ventral domain of the embryonic dorsal aorta. Proc Natl Acad Sci USA 104:9399–9403
Taylor E, Taoudi S, Medvinsky A (2010) Hematopoietic stem cell activity in the aorta-gonad-mesonephros region enhances after mid-day 11 of mouse development. Int J Dev Biol 54:1055–1060
Taoudi S, Gonneau C, Moore K, Sheridan JM, Blackburn CC, Taylor E, Medvinsky A (2008) Extensive hematopoietic stem cell generation in the AGM region via maturation of VE-cadherin + CD45 + pre-definitive HSCs. Cell Stem Cell 3:99–108
Kissa K, Herbomel P (2010) Blood stem cells emerge from aortic endothelium by a novel type of cell transition. Nature 464:112–115
Minot CS (1912) Development of the blood, the vascular system and the spleen. In: Keibel F, Mall FP (eds) Manual of human embryology. The Washington Square Press, Philadelphia
North T, Gu T-L, Stacy T, Wang Q, Howard L, Binder M, Marin-Padilla M, Speck NA (1999) Cbfa2 is required for the formation of the intra-aortic hematopoietic clusters. Development 126:2563–2575
Sheridan JM, Taoudi S, Medvinsky A, Blackburn CC (2009) A novel method for the generation of reaggregated organotypic cultures that permits juxtaposition of defined cell populations. Genesis 47:346–351
Gyevai A, Chapple PJ, Douglas WH (1978) Long-term maintenance of reaggregated hypothalamic cultures developed from embryonic rat hypothalamus: prostaglandin release during synaptogenesis in vitro. J Cell Sci 34:159–171
Bertrand JY, Giroux S, Golub R, Klaine M, Jalil A, Boucontet L, Godin I, Cumano A (2005) Characterization of purified intraembryonic hematopoietic stem cells as a tool to define their site of origin. Proc Natl Acad Sci USA 102:134–139
North TE, de Bruijn MFTR, Stacy T, Talebian L, Lind E, Robin C, Binder M, Dzierzak E, Speck NA (2002) Runx1 expression marks long-term repopulating hematopoietic stem cells in the midgestation mouse embryo. Immunity 16:661–672
Bertrand JY, Chi NC, Santoso B, Teng S, Stainier DYR, Traver D (2010) Haematopoietic stem cells derive directly from aortic endothelium during development. Nature 464:108–111
Boisset J-C, van Cappellen W, Andrieu-Soler C, Galjart N, Dzierzak E, Robin C (2010) In vivo imaging of haematopoietic cells emerging from the mouse aortic endothelium. Nature 464:116–120
Cho A, Courtman DW, Langille BL (1995) Apoptosis (programmed cell death) in arteries of the neonatal lamb. Circ Res 76:168–175
Lengerke C, Schmitt S, Bowman TV, Jang IH, Maouche-Chretien L, McKinney-Freeman S, Davidson AJ, Hammerschmidt M, Rentzsch F, Green JBA, Zon LI, Daley GQ (2008) BMP and Wnt specify hematopoietic fate by activation of the Cdx-Hox pathway. Cell Stem Cell 2:72–82
Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E (2007) Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proc Natl Acad Sci USA 104:20838–20843
Peeters M, Ottersbach K, Bollerot K, Orelio C, de Bruijn M, Wijgerde M, Dzierzak E (2009) Ventral embryonic tissues and Hedgehog proteins induce early AGM hematopoietic stem cell development. Development 136:2613–2621
Astorga J, Carlsson P (2007) Hedgehog induction of murine vasculogenesis is mediated by Foxf1 and Bmp4. Development 134:3753–3761
Kishigami S, Mishina Y (2005) BMP signaling and early embryonic patterning. Cytokine Growth Factor Rev 16:265–278
Gazzerro E, Smerdel-Ramoya A, Zanotti S, Stadmeyer L, Durand D, Economides AN, Canalis E (2007) Conditional deletion of gremlin causes a transient increase in bone formation and bone mass. J Biol Chem 282:31549–31557
Mitola S, Ravelli C, Moroni E, Salvi V, Leali D, Ballmer-Hofer K, Zammataro L, Presta M (2010) Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood 116:3677–3680
de Bruijn MFTR, Ma X, Robin C, Ottersbach K, Sanchez M-J, Dzierzak E (2002) Hematopoietic stem cells localize to the endothelial cell layer in the midgestation mouse aorta. Immunity 16:673–683
Stifani S, Ma Q (2009) “Runxs and regulations” of sensory and motor neuron subtype differentiation: implications for hematopoietic development. Blood Cells Mol Dis 43:20–26
Liakhovitskaia A, Lana-Elola E, Stamateris E, Rice DP, van’t Hof RJ, Medvinsky A (2010) the essential requirement for Runx1 in the development of the sternum. Dev Biol 340:539–546
Wang X, Blagden C, Fan J, Nowak SJ, Taniuchi I, Littman DR, Burden SJ (2005) Runx1 prevents wasting, myofibrillar disorganization, and autophagy of skeletal muscle. Genes Dev 19:1715–1722
Hoi CSL, Lee SE, Lu S-Y, McDermitt DJ, Osorio KM, Piskun CM, Peters RM, Paus R, Tumbar T (2010) Runx1 directly promotes proliferation of hair follicle stem cells and epithelial tumor formation in mouse skin. Mol Cell Biol 30:2518–2536
Holmes C, Stanford WL (2007) Concise review: stem cell antigen-1: expression, function, and enigma. Stem Cells 25:1339–1347
Kumano K, Chiba S, Kunisato A, Sata M, Saito T, Nakagami-Yamaguchi E, Yamaguchi T, Masuda S, Shimizu K, Takahashi T, Ogawa S, Hamada Y, Hirai H (2003) Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity 18:699–711
Hadland BK, Huppert SS, Kanungo J, Xue Y, Jiang R, Gridley T, Conlon RA, Cheng AM, Kopan R, Longmore GD (2004) A requirement for Notch1 distinguishes 2 phases of definitive hematopoiesis during development. Blood 104:3097–3105
Nakagawa M, Ichikawa M, Kumano K, Goyama S, Kawazu M, Asai T, Ogawa S, Kurokawa M, Chiba S (2006) AML/Runx1 rescues Notch1-null mutation-induced deficiency of para-aortic splanchnopleural hematopoiesis. Blood 108:3329–3334
Robert-Moreno A, Espinosa L, de la Pompa JL, Bigas A (2005) RBPjκ-dependent Notch function regulates Gata2 and is essential for the formation of intra-embryonic hematopoietic cells. Development 132:1117–1126
Landry J-R, Kinston S, Knezevic K, de Bruijn MFTR, Wilson N, Nottingham WT, Peitz M, Edenhofer F, Pimanda JE, Ottersbach K, Göttgens B (2008) Runx genes are direct targets of Scl/Tal1 in the yolk sac and fetal liver. Blood 111:3005–3014
Hayashi K, Abe N, Watanabe T, Obinata M, Ito M, Sato T, Habu S, Satake M (2001) Overexpression of AML1 transcription factor drives thymocytes into the CD8 single-positive lineage. J Immunol 167:4957–4965
Feng R, Desbordes SC, Xie H, Tillo ES, Pixley F, Stanley ER, Graf T (2008) PU.1 and C/EBPα/β converts fibroblasts into macrophage-like cells. Proc Natl Acad Sci USA 105:6057–6062
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676
Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Südhof TC, Wernig M (2010) Direct conversion of fibroblasts to functional neurons by defined factors. Nature 463:1035–1041
Marro S, Pang ZP, Yang N, Tsai M-C, Qu K, Chang HY, Südhof TC, Wernig M (2011) Direct lineage conversion of terminally differentiated hepatocytes to functional neurons. Cell Stem Cell 9:374–382
Giorgetti A, Marchetto MCN, Li M, Yu D, Fazzina R, Mu Y, Adamo A, Paramonov I, Cardoso JC, Monasterio MB, Bardy C, Cassiani-Ingoni R, Liu G-H, Gage FH, Izpisua Belmonte JC (2012) Cord blood-derived neuronal cells by ectopic expression of Sox2 and c-Myc. Proc Natl Acad Sci USA 109:12556–12561
Goyama S, Yamaguchi Y, Imai Y, Kawazu M, Nakagawa M, Asai T, Kumano K, Minati K, Ogawa S, Chiba S, Kurokawa M, Hirai H (2004) The transcriptionally active form of AML1 is required for hematopoietic rescue of the AML1-deficient embryonic para-aortic splanchnopleural (P-Sp) region. Blood 104:3558–3564
Sakai E, Kitajima K, Sato A, Nakano T (2009) Increase of hematopoietic progenitor and suppression of endothelial gene expression by Runx1 expression during in vitro ES differentiation. Exp Hematol 37:334–345
Johnson GR, Moore MAS (1975) Role of stem cell migration in initiation of mouse foetal liver haemopoiesis. Nature 258:726–728
Houssaint E (1981) Differentiation of the mouse hepatic primordium. II. Extrinsic origin of the haemopoietic cell line. Cell Differ 10:243–252
Gualdi R, Bossard P, Zheng M, Hamada Y, Coleman JR, Zaret KS (1996) Hepatic specification of the gut endoderm in vitro: cell signaling and transcriptional control. Genes Dev 10:1670–1682
Matsumoto K, Yoshitomi H, Rossant J, Zaret KS (2001) Liver organogenesis promoted by endothelial cells prior to vascular function. Science 294:559–563
Lammert E, Cleaver O, Melton D (2003) Role of endothelial cells in early pancreas and liver development. Mech Dev 120:59–64
Tavassoli M (1991) Embryonic and fetal hemopoiesis: an overview. Blood Cells 17:269–281
Chen MJ, Li Y, De Obaldia ME, Yang Q, Yzaguirre AD, Yamada-Inagawa T, Vink CS, Bhandoola A, Dzierzak E, Speck NA (2012) Erythroid/myeloid progenitors and hematopoietic stem cell originate from distinct populations of endothelial cells. Cell Stem Cell 9:541–552
Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A (2002) Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonization of the mouse embryonic liver. Development 129:4891–4899
Kieusseian A, Brunet de la Grange P, Burlen-Defranoux O, Godin I, Cumano A (2012) Immature hematopoietic stem cells undergo maturation in the fetal liver. Development 139:3521–3530
Collardeau-Frachon S, Scoazec J-Y (2008) Vascular development and differentiation during human liver organogenesis. Anat Rec 291:614–627
Rybtsov S, Sobiesiak M, Taoudi S, Souilhol S, Senserrich J, Liakhovitskaia A, Ivanovs A, Frampton J, Zhao S, Medvinsky A (2011) Hierarchical organization and early hematopoietic specification of the developing HSC lineage in the AGM region. J Exp Med 208:1305–1315
Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, Mehler MF, Conway SJ, Ng LG, Stanley ER, Samokhvalov IM, Merad M (2010) Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330:841–845
Couvelard A, Scoazec J-Y, Dauge M-C, Bringuier A-F, Potet F, Feldmann G (1996) Structural and functional differentiation of sinusoidal endothelial cells during liver organogenesis in human. Blood 87:4568–4580
Lichanska AM, Hume DA (2000) Origins and functions of phagocytes in the embryo. Exp Hematol 28:601–611
Bertrand JY, Jalil A, Klaine M, Jung S, Cumano A, Godin I (2005) Three pathways to mature macrophages in the early mouse yolk sac. Blood 106:3004–3011
Penaloza C, Lin L, Lockshin RA, Zakeri Z (2006) Cell death in development: shaping the embryo. Histochem Cell Biol 126:149–158
Silva MT, do Vale A, dos Santos NMN (2008) Secondary necrosis in multicellular animals: an outcome of apoptosis with pathogenic implications. Apoptosis 13:436–482
Potocnik AJ, Brakebusch C, Fassler R (2000) Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 12:653–663
Hirsh E, Iglesias A, Potocnik AJ, Hartmann U, Fässler R (1996) Impaired migration but not differentiation of haematopoietic stem cells in the absence of β1 integrins. Nature 380:171–175
Harris SG, Padilla J, Koumas L, Ray D, Phipps RP (2002) Prostaglandins as modulators of immunity. Trends Immunol 23:144–150
North TE, Goessling W, Walkley CR, Lengerke C, Kopani KR, Lord AM, Weber GJ, Bowman TV, Jang I-H, Grosser T, FitzGerald GA, Daley GQ, Orkin SH, Zon LI (2007) Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447:1007–1011
Nathan C (2002) Points of control in inflammation. Nature 420:846–852
Cabral GA (2005) Lipids as bioeffectors in the immune system. Life Sci 77:1699–1710
Orelio C, Haak E, Peeters M, Dzierzak E (2008) Interleukin-1 mediated hematopoietic cell regulation in the aorta-gonad-mesonephros region of the mouse embryo. Blood 112:4895–4904
Gurjar MV, Deleon J, Sharma RV, Bhalla RC (2001) Role of reactive oxygen species in IL-1 beta-stimulated sustained ERK activation and MMP-9 induction. Am J Physiol Heart Circ Physiol 281:H2568–H2574
Gong Y, Hart E, Shchurin A, Hoover-Plow J (2008) Inflammatory macrophage migration requires MMP-9 activation by plasminogen in mice. J Clin Invest 118:3012–3024
Hartner JC, Walkley CR, Lu J, Orkin SH (2009) ADAR1 is essential for the maintenance of hematopoiesis and suppression of interferon signaling. Nat Immunol 10:109–115
Quackenbush EJ, Wershil BK, Aguirre V, Gutierrez-Ramos J-C (2010) Eotaxin modulates myelopoiesis and mast cell development from embryonic hematopoietic progenitors. Blood 92:1887–1897
Kohchi C, Noguchi K, Tanabe Y, Mizuno D-I, Soma G-I (1994) Constitutive expression of TNF-α and -β genes in mouse embryo: roles of cytokines as regulator and effector on development. Int J Biochem 26:111–119
Flierl MA, Rittirsch D, Huber-Lang M, Sarma JV, Ward PA (2008) Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening Pandora’s box. Mol Med 14:195–204
Chen Y, Ke Q, Xiao Y-F, Wu G, Kaplan E, Hampton TG, Malek S, Min J-Y, Amende I, Morgan JP (2005) Cocaine and catecholamines enhance inflammatory cell retention in the coronary circulation of mice by upregulation of adhesion molecules. Am J Physiol Heart Circ Physiol 288:H2323–H2331
Guzik TJ, Korbut R, Adamek-Guzik T (2003) Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol 54:469–487
Krasnov P, Michurina T, Packer MA, Stasiv Y, Nakaya N, Moore KA, Drazan KE, Enikolopov G (2008) Neuronal nitric oxide synthase contributes to the regulation of hematopoiesis. Mol Med 14:141–149
Beste MT, Hammer DA (2008) Selectin catch-slip kinetics encode shear threshold adhesive behavior of rolling leukocytes. Proc Natl Acad Sci USA 105:20716–20721
Yago T, Zarnitsyna VI, Klopocki AG, McEver RP, Zhu C (2007) Transport governs flow-enhanced cell tethering through L-selectin at threshold shear. Biophys J 92:330–342
Kansas GS (1996) Selectins and their ligands: current concepts and controversies. Blood 88:3259–3287
Finger EB, Puri KD, Alon R, Lawrence MB, von Andrian UH, Springer TA (1996) Adhesion through L-selectin requires a threshold hydrodynamic shear. Nature 379:266–269
Nizet V, Johnson RS (2009) Interdependence of hypoxic and innate immune responses. Nat Rev Immunol 9:609–617
Taylor CT, Cummins EP (2009) The role of NF-κB in hypoxia-induced gene expression. Ann NY Acad Sci 1177:178–184
Dehne N, Brüne B (2009) HIF-1 in the inflammatory microenvironment. Exp Cell Res 315:1791–1797
Ahn KS, Aggarwal BB (2005) Transcription factor NF-κB. A sensor for smoke and stress signal. Ann N Y Acad Sci 1056:218–233
Orelio C, Harvey KN, Miles C, Oostendorp RAJ, van der Horn K, Dzierzak E (2004) The role of apoptosis in the development of AGM hematopoietic stem cells revealed by Bcl-2 overexpression. Blood 103:4084–4092
Wood W, Turmaine M, Weber R, Camp V, Maki RA, McKercher SR, Martin P (2000) Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 127:5245–5252
Halin C, Detmar M (2008) Chapter 1. Inflammation, angiogenesis and lymphangiogenesis. Methods Enzymol 445:1–25
Hoeffel G, Wang Y, Greter M, See P, Teo P, Malleret B, Leboeuf M, Low D, Oller G, Almeida F, Choy SHY, Grisotto M, Renia L, Conway SJ, Stanley ER, Chan JKY, Ng LG, Samokhvalov IM, Merad M, Ginhoux F (2012) Adult Langerhans cells derive predominantly from embryonic fetal liver monocytes with a minor contribution of yolk sac–derived macrophages. J Exp Med 209:1167–1181
Acknowledgments
This work was supported by Science and Technology Planning Project of Guangdong Province, China (2011A060901019).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Samokhvalov, I.M. Deconvoluting the ontogeny of hematopoietic stem cells. Cell. Mol. Life Sci. 71, 957–978 (2014). https://doi.org/10.1007/s00018-013-1364-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-013-1364-7