Summary
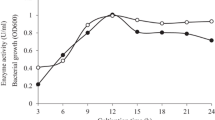
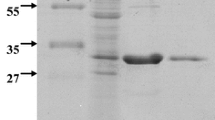
An alkalophilic bacterium producing high amounts of the cell-associated β-mannosidase and extracellular β-mannanase was isolated from soil. The isolate (AM-001) that grew well in alkaline pH media was identified as a strain of Bacillus sp. The optimal cultivation temperature for enzyme production was 31° C for β-mannosidase and 37° C for β-mannanase with the optimum production medium composed of 1% konjac powder, 0.2% yeast extract, 2% Polypepton, 0.1% K2HPO4, 0.02% MgSO4 · 7H2O and 0.5% Na2CO3. Optimum pH and temperature for β-mannosidase were 7.0 and 55° C, and for β-mannanase were 9.0 and 65° C.
Similar content being viewed by others
References
Araki T (1983) Purification and characterization of an endo-β-mannanase from Aeromonas sp. F-25. J Fac Agr Kyushu Univ 27 (3.4):89–98
Araki T, Kitamikado M (1982) Purification and characterization of a novel exo-β-mannanase from Aeromonas sp. F-25 J Biochem 91:1181–1186
Bouquelet S, Spik G, Montreuil J (1978) Properties of a β-mannosidase from Aspergillus niger. Biochim Biophys Acta 552:521–530
Buchanan RE, Gibbons VE (1974) Bergey's manual of determinative bacteriology, 8th edn. Williams and Wilkins, Baltimore
Civas A, Bverhard, Dizet PL, Petek F (1984) Glycosidase induced in Aspergillus tamarii secreted α-D-galactosidase and β-D-mannanase. Biochem J 219:857–863
Emi S, Fukumoto J, Yamamoto T (1972) Crystallization and some properties of mannanase Agric Biol Chem 36:991–1001
Eriksson KE, Winnel M (1968) Purification and characterization of a fungal β-mannanase. Acta Chemica Scandinavia 22:1924–1934
Hashimoto Y, Fukumoto J (1969a) Studies on the enzyme treatment of coffee beans. Part I. Purification of mannanase of Rhizopus niveus and its action on coffee mannan. Nippon Nogeikagaku Kaishi 43(5):317–322
Hashimoto Y, Fukumoto J (1969b) Studies on the enzyme treatment of coffee beans. Part II. Purification and properties of β-mannosidase of Rhizopus niveus. Nippon Nogeikagaku Kaishi 43(8):564–569
Horikoshi K (1971a) Production of alkaline enzymes by alkalophilic microorganisms. Part I. Alkaline protease produced by Bacillus No. 221. Agric Biol Chem 35:1407–1414
Horikoshi K (1971b) Production of alkaline enzymes by alkalophilic microorganisms. Part II. Alkaline amylase produced by Bacillus No. A-40-2. Agric Biol Chem 35:1783–1791
Horikoshi K, Akiba T (1982) Alkalophilic microorganisms. Japan scientific Societies Press, Tokyo
Nakamura N, Horikoshi K (1976) Purification and properties of cyclodextrin glycosyltransferase of an alkalophilic Bacillus sp. Agric Biol Chem 40:935–941
Nelson N (1944) A photometric adaptation of the Somogyi method for the determination of glucose. J Biol Chem 153:375–380
Saiki T (1975) Allosteric enzymes of thermophilic bacteria. Protein, Nucleic acid and Enzyme 20(3):188–193
Somogyi M (1945) A new reagent for the determination of sugars. J Biol Chem 160:61–68
Sugiyama N, Shimahara H, Andoh T (1972) Studies on mannan and related compounds. I. The purification of konjac mannan. Bull Chem Soc Jap 45:561–563
Takahashi R, Kusakabe I, Kobayashi H, Murakami K, Maekawa A, Suzuki I (1984) Purification and some properties of mannanase from Streptomyces sp. Agric Biol Chem 48(9):2189–2195
Tamaoka J, Komagata K (1984) Determination of DNA base composition by reversed-phase high-performance liquid chromatography. FEMS Microbiol Letter 25:125–128
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Akino, T., Nakamura, N. & Horikoshi, K. Production of β-mannosidase and β-mannanase by an alkalophilic Bacillus sp.. Appl Microbiol Biotechnol 26, 323–327 (1987). https://doi.org/10.1007/BF00256662
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00256662