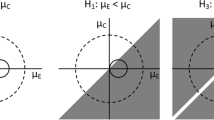
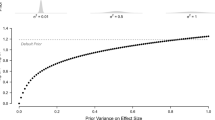
Abstract
Based on the concept of repeated significance tests, an empirical study may be planned in subsequent stages. Group sequential test procedures offer the possibility of performing the study with a fixed number of observations per stage. At least, the number of observations must be chosen independently of the observed data. In adaptive group sequential test procedures, the number of observations can be changed during the course of the study using all results observed so far. In this article, the basic concepts of these two designs are reviewed. Recent developments in adaptive designs are outlined and potential fields of application are given.
Similar content being viewed by others
References
Armitage, P. (1975).Sequential Medical Trials. Blackwell, Oxford, second edition.
Armitage, P., McPherson, C. K., Rowe, B. C. (1969). Repeated significance tests on accumulating data.J. Roy. Stat. Soc. A 132, 235–244.
Banik, N., Köhne, K., Bauer, P. (1996). On the power of Fisher’s combination test for two stage sampling in the presence of nuisance parameters.Biometrical J. 38, 25–37.
Bauer, M., Bauer, P., Budde, M. (1998). A simulation program for adaptive two-stage designs.Computational Statistics & Data Analysis 26, 351–371.
Bauer, P. (1989a). Multistage testing with adaptive designs.Biom. und Inform. in Med. und Biol. 20, 130–148.
Bauer, P. (1989b). Sequential tests of hypotheses in consecutive trials.Biometrical J. 31, 663–676.
Bauer, P. (1992). The choice of sequential boundaries based on the concept of power spending.Biom. und Inform. in Med. und Biol. 23, 3–15.
Bauer, P., Kieser, M. (1999). Combining different phases in the development of medical treatments within a single trial.Statistics in Medicine 18, 1833–1848.
Bauer, P., Köhne, K. (1994). Evaluation of experiments with adaptive interim analyses.Biometrics 50, 1029–1041.
Bauer, P., Röhmel, J. (1995). An adaptive method for establishing a dose-response relationship.Statistics in Medicine 14, 1595–1607.
Bauer, P., Scheiber, V., Wohlzogen, F. X. (1986).Sequentielle statistische Verfahren. Gustav Fisher Verlag, Stuttgart.
Birkett, M. A., Day, S. J. (1994). Internal pilot studies for estimating sample size.Statistics in Medicine 13, 2455–2463.
Brannath, W., Posch, M., Bauer, P. (1999). Recursive combination tests.Submitted.
Brittain, E. H., Bailey, K. R. (1993). Optimization of multistage testing times and critical values in clinical trials.Biometrics 49, 763–772.
Chang, M. N., Hwang, I. K., Shih, W. J. (1998). Group sequential designs using both type I and type II error probability spending functions.Comm. Stat.—Theory Meth. 27, 1323–1339.
Chi, G. Y. H., Liu, Q. (1999). The attractiveness of the concept of a prospectively designed two-stage clinical trial.Journal of Biopharmaceutical Statistics 9, 537–547.
Cui, L., Hung, H. M. J., Wang, S.-J. (1999). Modification of sample size in group sequential clinical trials.Biometrics 55, 853–857.
DeMets, D. L., Ware, J. H. (1980). Group sequential methods for clinical trials with a one-sided hypothesis.Biometrika 67, 651–660.
DeMets, D. L., Ware, J. H. (1982). Asymmetric group sequential boundaries for monitoring clinical trials.Biometrika 69, 661–663.
Dodge, H. F., Romig, H. G. (1929). A method of sampling inspection.Bell. System Technical J. 8, 613–631.
Emerson, S. S. (1996). Statistical packages for group sequential methods.The American Statistician 50, 183–192.
Emerson, S. S., Fleming, T. R. (1989). Symmetric group sequential test designs.Biometrics 45, 905–923.
Fisher, L. D. (1998). Self-designing clinical trials.Statistics in Medicine 17, 1551–1562.
Fleming, T. R., Harrington, D. P., O’Brien, P. C. (1984). Designs for group sequential trials.Contr. Clin. Trials 5, 348–361.
Follmann, D. A., Proschan, M. A., Geller, N. L. (1994). Monitoring pairwise comparisons in multi-armed clinical trials.Biometrics 50, 325–336.
Ghosh, B. K. (1970).Sequential Tests of Statistical Hypotheses. Addison-Wesley, Reading, Massachusetts.
Gould, A. L. (1992). Interim analysis for monitoring clinical trials that do not materially affect the type I error rate.Statistics in Medicine 11, 53–66.
Gould, A. L. (1995). Planning and revising the sample size for a trial.Statistics in Medicine 14, 1039–1051.
Gould, A. L. (1997). Issues in blinded sample size re-estimation.Comm. Stat.—Sim. Comp. 26, 1229–1239.
Gould, A. L., Pecore, V. J. (1982). Group sequential methods for clinical trials allowing early acceptance of H0 and incorporating costs.Biometrika 69, 75–80.
Gould, A. L., Shih, W. J. (1992). Sample size re-estimation without unblinding for normally distributed outcomes with unknown variance.Comm. Stat.—Theory Meth. 21, 2833–2853.
Gould, A. L., Shih, W. J. (1998). Modifying the design of ongoing trials without unblinding.Statistics in Medicine 17, 89–100.
Hayre, L. S. (1985). Group sequential sampling with variable group sizes.J. R. Statist. Soc. B. 47, 90–97.
Hedges, L. V., Olkin, I. (1985).Statistical Methods for Meta Analysis. New York: Akademic Press.
Hwang, I. K., Shih, W. J., DeCani, J. S. (1990). Group sequential designs using a family of Type I error probability spending functions.Statistics in Medicine 9, 1439–1445.
Jennison, C. (1987). Efficient group sequential tests with unpredictable group sizes.Biometrika 74, 155–165.
Jennison, C., Turnbull, B. (1991a). Exact calculations for sequential: t, chi-square and F tests.Biometrika 78, 133–141.
Jennison, C., Turnbull, B. (1991b). Group sequential tests and repeated confidence intervals.Handbook of Sequential Analysis (Ghosh Sen, eds.) pages 283–311.
Jennison, C., Turnbull, B. W. (1999).Group Sequential Methods with Applications to Clinical Trials. Chapman & Hall, Boca Raton, London, New York, Washington, D.C.
Kieser, M., Bauer, P., Lehmacher, W. (1999). Inference on multiple endpoints in clinical trials with adaptive interim analyses.Biometrical J. 41, 261–277.
Kieser, M., Friede, T. (2000). Re-calculating the sample size in internal pilot study designs with control of the type I error rate.Statistics in Medicine, in press.
Kim, K., Boucher, H., Tsiatis, A. A. (1995). Design and analysis of group sequential logrank tests in maximum duration versus information trials.Biometrics 51, 988–1000.
Kim, K., DeMets, D. L. (1987). Design and analysis of group sequential tests based on the Type I error spending rate function.Biometrika 74, 149–154.
Köpcke, W. (1984).Zwischenauswertungen und vorzeitiger Abbruch von Therapiestudien. Springer, Berlin, Heidelberg, New York, Tokyo.
Köpcke, W. (1989). Analyses of group sequential clinical trials.Controlled Clinical Trials 10, 222–230S.
Lan, K. K. G., DeMets, D. L. (1983). Discrete sequential boundaries for clinical trials.Biometrika 70, 659–663.
Lan, K. K. G., DeMets, D. L. (1989). Group sequential procedures: Calender versus information time.Statistics in Medicine 8, 1191–1198.
Lan, K. K. G., Reboussin, D. M., DeMets, D. L. (1994). Information and information fractions for design and sequential monitoring of clinical trials.Comm. Stat.—Theory Meth. 23, 403–420.
Lehmacher, W., Kieser, M., Hothorn, L. (2000). Sequential and multiple testing for dose-response analysis.Drug Information J., in press.
Lehmacher, W., Wassmer, G. (1999). Adaptive sample size calculations in group sequential trials.Biometrics 55, 1286–1290.
Li, Z., Geller, N. L. (1991). On the choice of times for data analysis in group sequential trials.Biometrics 47, 745–750.
Liu, W. (1995). A group sequential procedure for all-pairwise comparisons of k treatments based on range statistics.Biometrics 51, 946–955.
McPherson, C. K., Armitage, P. (1971). Repeated significance tests on accumulating data when the null hypothesis is not true.J. Roy. Stat. Soc. A 134, 15–25.
McPherson, K. (1982). On choosing the number of interim analyses in clinical trials.Statistics in Medicine 1, 25–36.
Müller, H.-H., Schäfer, H. (1999a). Adaptive group sequential designs for clinical trials: Combining the advantages of adaptive and of classical group sequential approaches.Submitted.
Müller, H.-H., Schäfer, H. (1999b). Optimization of testing times and critical values in sequential equivalence testing.Statistics in Medicine 18, 1769–1788.
O’Brien, P. C., Fleming, T. R. (1979). A multiple testing procedure for clinical trials.Biometrics 35, 549–556.
Pampallona, S., Tsiatis, A. A. (1994). Group sequential designs for one-sided and two-sided hypothesis testing with provision for early stopping in favour of the null hypothesis.J. Stat. Plan. Inf. 42, 19–35.
Pampallona, S., Tsiatis, A. A., Kim, K. (1995). Spending functions for the type I and type II error probabilities of group sequential tests.Manuscript.
Pigeot, I. (1999). Basic concepts of multiple tests—a survey.Statistical Papers.
Pocock, S. J. (1977). Group sequential methods in the design and analysis of clinical trials.Biometrika 64, 191–199.
Pocock, S. J. (1982). Interim analyses for randomized clinical trials: the group sequential approach.Biometrics 38, 153–162.
Posch, M., Bauer, P. (1999). Adaptive two stage designs and the conditional error function.Biometrical J. 41, 689–696.
Proschan, M. A. (1999). A multiple comparison procedure for three- and four-armed controlled clinical trials.Statistics in Medicine 18, 787–798.
Proschan, M. A., Follmann, D. A., Waclawiw, M. A. (1992). Effects on assumption violations on type I error rate in group sequential monitoring.Biometrics 48, 1131–1143.
Proschan, M. A., Hunsberger, S. A. (1995). Designed extension of studies based on conditional power.Biometrics 51, 1315–1324.
SAS Institute Inc. (1995).SAS/IML Software: Changes and Enhancements through Release 6. 11. Cary, NC: SAS Institute Inc.
Schäfer, H., Müller, H.-H. (1999). A general statistical method for design changes during the course of an experiment.Submitted.
Shen, Y., Fisher, L. (1999). Statistical inference for self-designing clinical trials with a one-sided hypothesis.Biometrics 55, 190–197.
Šidák, Z. (1967). Rectangular confidence regions for the means of multivariate normal distributions.J. Amer. Stat. Ass. 62, 626–633.
Siegmund, D. (1985).Sequential Analysis. Springer, New York, Berlin, Heidelberg, Tokyo.
Slepian, D. (1962). The one-sided barrier problem for Gaussian noise.Bell. System Technical Journal 41, 463–501.
Slud, E., Wei, L. J. (1982). Two-sample repeated significance tests based on the modified Wilcoxon statistic.J. Amer. Stat. Ass. 77, 862–868.
Sonnemann, E. (1991). Kombination unabhängiger Tests.In: Vollmar, J. (ed.): Biometrie in der chemisch-pharmazeutischen Industrie 4, 91–112.
Stein, C. (1945). A two-sample test for a linear hypothesis whose power is independent of the variance.Ann. Math. Statist. 16, 243–258.
Tang, D.-I., Geller, N. L. (1999). Closed testing procedures for group sequential clinical trials with multiple endpoints.Biometrics 55, 1188–1192.
Turnbull, B. W. (1997). Group sequential tests.Encyclopedia of Statistical Sciences (Kotz, Read, Banks eds).
Wald, A. (1947).Sequential Analysis. Wiley, New York.
Wang, S. K., Tsiatis, A. A. (1987). Approximately optimal one-parameter boundaries for group sequential trials.Biometrics 43, 193–199.
Wassmer, G. (1997). A technical note on the power determination for Fisher’s combination test.Biometrical J. 39, 831–838.
Wassmer, G. (1998). A comparison of two methods for adaptive interim analyses in clinical trials.Biometrics 54, 696–705.
Wassmer, G. (1999a). Group sequential monitoring with arbitrary inspection times.Biometrical J. 41, 197–216.
Wassmer, G. (1999b). Multistage adaptive test procedures based on Fisher’s product criterion.Biometrical J. 41, 279–293.
Wassmer, G. (1999c).Statistische Testverfahren für gruppensequentielle und adaptive Pläne in klinischen Studien. Theoretische Konzepte und deren praktische Umsetzung mit SAS. Verlag Alexander Mönch, Köln.
Wetherill, G. B. (1975).Sequential Methods in Statistics. Chapman and Hall, London.
Whitehead, J. (1997).The Design and Analysis of Sequential Clinical Trials. Wiley, New York, revised 2nd edition.
Wittes, J., Brittain, E. (1990). The role of internal pilot studies in increasing the efficiency of clinical trials.Statistics in Medicine 9, 65–72.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wassmer, G. Basic concepts of group sequential and adaptive group sequential test procedures. Statistical Papers 41, 253–279 (2000). https://doi.org/10.1007/BF02925923
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF02925923