Abstract
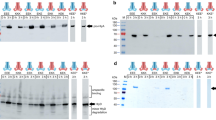
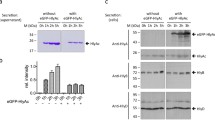
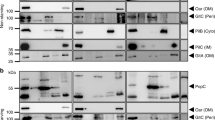
One of the strategies used by Gram-negative bacteria to secrete proteins across the two membranes which delimit the cells, issec independent and dedicated to proteins lacking an N-terminal signal peptide. It depends on ABC protein-mediated exporters, which consist of three cell envelope proteins: two inner membrane proteins: an ATPase (the ABC protein), a membrane fusion protein (MFP) and an outer membrane polypeptide.Erwinia chrysanthemi metalloproteinases B and C, andSerratia marcescens hemoprotein HasA are secreted by such homologous pathways and interact with the ABC protein. Interaction between the ABC protein and its substrate has also been evidenced by studies on proteinase and HasA hybrid transporters obtained by combining components from each system. Association between hemoprotein HasA and the three exporter/secretion proteins was demonstrated by affinity chromatography on hemin agarose on which the substrate remained bound with the three secretion proteins. The three component association was ordered and substrate binding was required for the formation of this multiprotein complex.
Similar content being viewed by others
References
Akatsura H., Kawai E., Omori K., Shibatani T.: The three geneslipB, lipC, lipD involved in the extracellular secretion of theSerratia marcescens lipase which lacks an N-terminal signal peptide.J. Bacteriol. 177, 6381–6389 (1995).
Binet R., Wandersman C.: Protein secretion by hybrid bacterial ABC-transporters: specific functions of the membrane ATPase and the membrane fusion protein.EMBO J. 14, 2298–2306 (1995).
Binet R., Wandersman C.: Cloning of theSerratia marcescens hasF gene encoding the HasABC exporter outer membrane component, a TolC analoque.Mol Microbiol. 22, 265–273 (1996).
Boos W., Lucht J.M.: Periplasmic binding protein-dependent ABC transporters, pp. 1175–1209 in F.C. Neidhartet al., (Eds):Escherichia coli and Salmonella, Cellular and Molecular Biology. Amer. Soc. Microbiol. Press, Washington (DC) 1996.
Delepelaire P.: PrtD, the integral membrane ATP-binding cassette component of theErwinia chrysanthemi metalloprotease secretion system, exhibits a secretion signal-regulated ATPase activity.J. Biol. Chem. 269, 27952–27957 (1994).
Delepelaire P., Wandersman C.: Protein secretion in Gram-negative bacteria. The extracellular metalloprotease B fromErwinia chrysanthemi, contains a C-terminal secretion signal analogous to that ofEscherichia coli α-haemolysin.J. Biol. Chem. 265, 9083–9089 (1990).
Dinh T., Paulsen I.T., Saier M.H.J.: A family of extracytoplasmic proteins that allow transport of large molecules across the outer membranes of Gram-negative bacteria.J. Bacteriol. 176, 3825–3831 (1994).
Duong F., Soscia C., Lazdunski A., Murgier M.: ThePseudomonas fluorescens lipase has a C-terminal secretion signal and is secreted by a three-component bacterial ABC-exporter system.Mol. Microbiol. 11, 1117–1126 (1994).
Fath M.J., Kolter R.: ABC transporters: bacterial exporters.Microbiol. Rev. 57, 995–1017 (1993).
Fath M.J., Skvirsky R.C., Kolter R.: Functional complementation between bacterial MDR-like export systems: Colicin V, α-haemolysin andErwinia proteases.J. Bacteriol. 173, 7549–7556 (1991).
Ghigo J.M., Wandersman C.: A carboxyl-terminal four-amino acid motif is required for secretion of the metalloprotease PrtG through theErwinia chrysanthemi protease secretion pathway.J. Biol. Chem. 269, 8979–8985 (1994).
Gottesman M.M., Hrycyna C.A., Germann U.A., Pastan I.: Genetic analysis of the multidrug transporter.Ann. Rev. Genet. 29, 607–649 (1995).
Gottesman M.M., Pastan I.: Biochemistry of multidrug resistance mediated by the multidrug transporter.Ann. Rev. Biochem. 62, 385–427 (1993).
Guzzo J., Duong F., Wandersman C., Murgier M., Lazdunski A.: The secretion genes ofPseudomonas aeruginosa alkaline protease are functionally related to those ofErwinia chrysanthemi proteases andEscherichia coli α-haemolysin.Mol. Microbiol. 5, 447–453 (1991).
Higgins C.F.: ABC transporters—from microorganisms to man.Ann. Rev. Cell Biol. 8, 67–113 (1992).
Koronakis V., Stanley P., Koronalis E., Hughes C.: The HlyB/HlyD-dependent secretion of toxins by Gram-negative bacteria.FEMS Microbiol. Immunol. 105, 45–54 (1992).
Létoffé S., Delepelaire P. Wandersman C.: Protein secretion in Gram-negative bacteria: Assembly of the three components of ABC protein-mediated exporters is ordered and promoted by substrate binding.EMBO J., in press (1996).
Létoffé S., Delepelaire P., Wandersman C.: Protease secretion byErwinia chrysanthemi: the specific secretion fonctions are analogous to those ofE. coli α-haemolysin.EMBO J. 9, 1375–1382 (1990).
Létoffé S., Ghigo J.M., Wandersman C.: Iron acquisition from heme and hemoglobin bySerratia marcescens extracellular protein.Proc. Nat. Acad. Sci. USA 91, 9876–9880 (1994a).
Létoffé S., Ghigo J.M., Wandersman C.: Secretion of theSerratia marcescens HasA protein by an ABC transporter.J. Bacteriol. 176, 5372–5377 (1994b).
Létoffé S., Wandersman C.: Secretion of CyaA-PrtB and HlyA-PrtB fusions proteins inEscherichia coli: Involvement of glycinerich repeat domain ofErwinia chrysanthemi protease B.J. Bacteriol. 174, 4920–4927 (1992).
Mackman N., Nicaud J.M., Gray V., Holland I.B.: Secretion of hemolysin byEscherichia coli, pp. 159–181 inCurr. Topics Microbiol. Immunol, Vol. 125. Springer-Verlag, Berlin-Heidelberg 1986.
Ménard R., Sansonetti P., Parsot C.: The secretion of theShigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD.EMBO J. 13, 5293–5302 (1994).
Murphy C.K., Beckwith J.: Export of proteins to the cell envelope inEscherichia coli, pp. 967–978 in F.C. Neidhartet al. (Eds):Escherichia coli and Salmonella, Cellular and Molecular Biology. Amer. Soc. Microbiol. Press, Washington (DC) 1996.
Nakahama K., Yosomura K., Marumoto R., Kikuchi M., Sook Lee I., Hase T., Matsubara H.: Cloning and sequencing ofSerratia protease gene.Nucl. Acids Res. 14, 5843–5855 (1986).
Pugsley A.P., Possot O.: The general secretory pathway ofKlebsiella oxytoca: no evidence for relocalization or assembly of pilin like PulG protein into a multiprotein complex.Mol. Microbiol. 10, 665–674 (1993).
Riordan J.R., Rommens J.M., Kerein B.S., Alon N., Rozmahel R. et al.: Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA.Science 245, 1066–1073 (1989).
Wandersman C.: Secretion across the bacterial outer membrane, pp. 955–967 in F.C. Neidhartet al. (Eds):Escherichia coli and Salmonella typhimurium, Cellular and Molecular Biology. Amer. Soc. Microbiol. Press, Washington (DC) 1996.
Wandersman C., Delepelaire P.: TolC, anEscherichia coli outer membrane protein required for hemolysin secretion.Proc. Nat. Acad. Sci. USA 87, 4776–4780 (1990).
Welch R.A.: Pore-forming cytolysins of Gram-negative bacteria.Mol. Microbiol. 60, 101–124 (1991).
Wolff N., Ghigo J.M., Delepelaire P., Wandersman C., Delepierre M.: C-Terminal secretion signal of anErwinia chrysanthemi protease secreted by a signal peptide-independent pathway: proton NMR and CD conformational studies in membranemimetic environments.Biochemistry 33, 6792–6801 (1994).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Binet, R., Létoffé, S., Ghigo, J.M. et al. Protein secretion by gram-negative bacterial ABC exporters. Folia Microbiol 42, 179–183 (1997). https://doi.org/10.1007/BF02818975
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02818975