Abstract
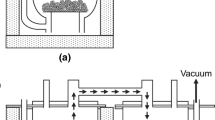
The reduction of high-grade hematite ore in hydrogen has been investigated. There is an unusual temperature effect for small granules with a dip in the rate at about 700°C, similar to those reported by previous investigators for different types of iron oxides. The particlesize effect on the time of reduction suggests that there are three major limiting rate-controlling processes: i) uniform internal reduction, ii) limiting mixed control and iii) gas diffusion in porous iron layer. Processes (ii) and (iii) are special cases of a so-called topochemical mode of reduction associated with the formation of product layers. Unidirectional reduction experiments revealed the significant role played by gas diffusion in porous iron layer as a rate-controlling process. The effective H2-H2O diffusivity in porous iron derived from, the reduction data is found to decrease markedly with decreasing reduction temperature. This is consistent with the fracture surfaces of porous iron as viewed by scanning electron microscopy. The present interpretation of the rate of reduction of hematite ore is found to apply equally well to previously published data on the hydrogen-reduction of natural and synthetic hematite pellets.
Similar content being viewed by others
References
W. M. McKewan:Steelmaking, The Chipman Conference, pp. 141–55, MIT Press, 1965;Trans. TMS-AIME, 1960, vol. 218, pp. 2–6.
J. M. Quets, M. E. Wadsworth, and J. R. Lewis:Trans. TMS-AIME, 1960, vol. 218, pp. 545–50;Trans. TMS-AIME, 1961, vol. 221, pp. 1186–93.
N. J. Themelis and W. H. Gauvin:A.I.Ch.E.J., 1962, vol. 8, pp. 437–44;Trans. TMS-AIME, 1963, vol. 227, pp. 290–300.
N. A. Warner:Trans. TMS-AIME, 1964, vol. 230, pp. 163–78.
R. H. Spitzer, F. S. Manning, and W. O. Philbrook:Trans. TMS-AIME, 1966, vol. 236, pp. 726–42.
R. G. Olsson and W. M. McKewan:Trans. TMS-AIME, 1966, vol. 236, pp. 1518–22.
R. G. Olsson and W. M. McKewan:Met. Trans., 1970, vol. 1, pp. 1507–12.
T. L. Joseph:Trans. AIME, 1936, vol. 120, pp. 72–90.
R. P. Smith: Edgar C. Bain Laboratory for Fundamental Research, U.S. Steel Corp., Monroeville, Pa., private communication.
J. O. Edstrom:J. Iron Steel Inst., 1953, vol. 175, pp. 289–304.
M. C. Udy and C. H. Lorig:Trans. AIME, 1943, vol. 354, pp. 162–81.
J. Henderson:J. Australian Inst. Metals, 1962, vol. 7, pp. 115–21;AIME Conf. 8 Phys. Chem. Process Met., pp. 671–85, Interscience, New York, 1961.
W. Wenzel, H. W. Gudenau, and M. Ponthenkandath:Aufbereitungs. Technik, 1970, no. 8, pp. 492–94.
R. H. Tien and E. T. Turkdogan:Carbon, In Press.
E. W. Thiele:Ind. Eng. Chem., 1939, vol. 31, pp. 916–20.
B. B. L. Seth and H. U. Ross:Trans. TMS-AIME, 1965, vol. 233, pp. 180–85.
J. Crank:The Mathematics of Diffusion, Oxford University Press, 1956.
J. A. Currie:Brit. J. Appl. Phys., 1960, vol. 11, pp. 318–24.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Turkdogan, E.T., Vinters, J.V. Gaseous reduction of iron oxides: Part I. Reduction of hematite in hydrogen. Metall Trans 2, 3175–3188 (1971). https://doi.org/10.1007/BF02814970
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02814970